Dual nonradical pathways in enhanced PDS activation by Fe@BC-S: Fe7S8 mediated the relationship between 1O2 and electron transfer
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
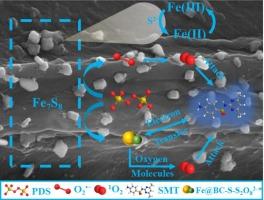
Biochar (BC)-based catalysts have been widely utilized in the activation of peroxydisulfate (PDS), representing an effective water treatment technique for decontamination purposes. However, the mechanisms of PDS activation by S and Fe co-doped biochar hybrid catalysts were still ambiguous. Therefore, Fe-S biochar by exogenous doping (Fe@BC-S) was prepared to activate PDS for sulfamethazine (SMT) degradation, and Fe7S8 sites were formed in Fe@BC-S acting as the main active center. The SMT degradation efficiency within 50 min in Fe@BC-S/PDS system (95.3 %) was much higher than that in Fe@BC/PDS system (62.5 %). It was found that the dual nonradical pathways by 1O2 and electron transfer dominated in the more efficient PDS activation and SMT degradation. The detailed mechanisms in the activation process could be determined to: (i) Successive 1O2 generation mediated via Fe(II)/Fe(III) redox cycle with a steady-state concentration of 5.66 × 10-13 M. And the 1O2 was derived from not only reaction process of PDS → O2·-→ 1O2, but also the oxidation of the oxygen molecules in the sub-stable complexes; (ii) The doping of S promoted the electrons transfer capability, enabling the formation of sub-stable complexes between Fe@BC-S and PDS through outer-sphere interactions, with Fe7S8 acting as an electron intermediate mediating the electron transfer process from the pollutants to PDS. Further, the dual nonradical pathways by 1O2 and electron transfer selectively attacked the electron-rich sites of SMT. In addition, Fe@BC-S exhibited high environmental adaptability and stability. This work suggested a new strategy for biochar modification and provided new insights into the enhanced water decontamination by nonradical pathways.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


