Mitochondrial respiratory complex IV deficiency recapitulates amyotrophic lateral sclerosis
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Amyotrophic lateral sclerosis (ALS) is categorized into ~10% familial and ~90% sporadic cases. While familial ALS is caused by mutations in many genes of diverse functions, the underlying pathogenic mechanisms of ALS, especially in sporadic ALS (sALS), are largely unknown. Notably, about half of the cases with sALS showed defects in mitochondrial respiratory complex IV (CIV). To determine the causal role of this defect in ALS, we used transcription activator-like effector-based mitochondrial genome editing to introduce mutations in CIV subunits in rat neurons. Our results demonstrate that neuronal CIV deficiency is sufficient to cause a number of ALS-like phenotypes, including cytosolic TAR DNA-binding protein 43 redistribution, selective motor neuron loss and paralysis. These results highlight CIV deficiency as a potential cause of sALS and shed light on the specific vulnerability of motor neurons, marking an important advance in understanding and therapeutic development of sALS. Cheng et al. identify a mitochondrial complex IV (CIV) deficiency in the brains of patients with sporadic amyotrophic lateral sclerosis (ALS). They demonstrate that defects in mitochondrial CIV induce ALS-like phenotypes in rats and highlight CIV deficiency as a potential risk factor and therapeutic target for ALS.

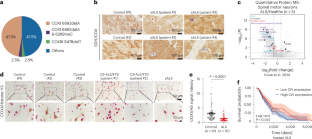
线粒体呼吸复合体IV缺乏是肌萎缩性侧索硬化症的表现
肌萎缩性脊髓侧索硬化症(ALS)分为约 10% 的家族性病例和约 90% 的散发性病例。家族性渐进性脊髓侧索硬化症是由许多功能各异的基因突变引起的,而渐进性脊髓侧索硬化症,尤其是散发性渐进性脊髓侧索硬化症(sALS)的潜在致病机制在很大程度上尚属未知。值得注意的是,约有一半的散发性 ALS 病例显示线粒体呼吸复合体 IV(CIV)存在缺陷。为了确定这一缺陷在 ALS 中的因果作用,我们使用基于转录激活剂样效应器的线粒体基因组编辑技术,在大鼠神经元中引入 CIV 亚基的突变。我们的研究结果表明,神经元 CIV 缺乏足以导致一系列类似 ALS 的表型,包括细胞膜 TAR DNA 结合蛋白 43 的重新分布、选择性运动神经元缺失和瘫痪。这些结果突显了 CIV 缺乏是导致渐冻人症的潜在原因之一,并揭示了运动神经元的特殊脆弱性,标志着在了解渐冻人症和开发治疗方法方面取得了重要进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


