Pd-catalyzed domino Heck cyclization/cross-coupling of indoles with β-chlorovinyl ketones: synthesis of furan-bearing indolo[2,1-α]isoquinolines with antifungal activity †
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein, a palladium-catalyzed cross-coupling cascade cyclization of alkene-tethered indoles and (E)-β-chlorovinyl ketones, providing access to various furan-bearing indolo[2,1-α]isoquinolines, is reported. The reaction enables the rapid construction of one C–O bond and two C–C bonds to form bis-heterocycles. Furthermore, these furan-bearing indolo[2,1-α]isoquinolines exhibited certain antifungal activity in vitro. Notably, bis-heterocycles (EC50 = 16.5014 μg mL−1) against Botryosphaeria dothidea and (EC50 = 18.2751 μg mL−1) against Rhizoctonia solani were identified to have good inhibitory effects.
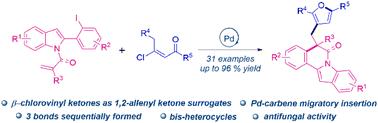
pd催化吲哚与β-氯乙烯基酮的多米诺Heck环化/交叉偶联:合成具有抗真菌活性的含呋喃吲哚[2,1-α]异喹啉。
本文报道了钯催化的烯系吲哚和(E)-β-氯乙烯基酮的交叉偶联级联环化反应,从而获得了多种含呋喃的吲哚[2,1-α]异喹啉。该反应可以快速构建一个C-O键和两个C-C键,形成双杂环。此外,这些含呋喃的吲哚[2,1-α]异喹啉在体外表现出一定的抗真菌活性。值得注意的是,双杂环3da (EC50 = 16.5014 μ mL-1)和3ar (EC50 = 18.2751 μ mL-1)对番茄根丝胞菌均有较好的抑制作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


