Influence of Dihydrophenazine Photoredox Catalyst Excited State Character and Reduction Potentials on Control in Organocatalyzed Atom Transfer Radical Polymerization
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
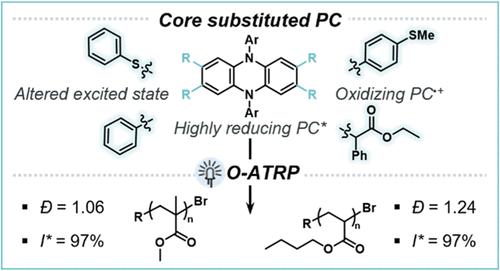
The development of N,N-diaryl dihydrophenazine organic photoredox catalysts (PCs) has enabled numerous examples of organocatalyzed atom transfer radical polymerization (O-ATRP) of methyl methacrylate (MMA) monomer to polymers with low dispersity (Đ < 1.30) and near-unity initiator efficiency (I* ∼ 100%), as well as small molecule synthesis. In this work, we investigate the influence of core substitution (CS) by alkyl, aryl, and heteroatom groups on singlet excited state reduction potential (ES1°*). We observe that a highly reducing ES1°* is in part a result of a locally excited (LE)-dominated hybridized local and charge transfer (HLCT) excited state in CS PCs, which is influenced by the identity of the core substituent. Additionally, the PCs that possess a LE-dominated HLCT character maintain a relatively oxidizing PC radical cation oxidation potential (E1/2) for deactivation in O-ATRP compared to fully LE PCs reported in prior work. For example, a thiophenol core substituted (heteroatom CS, HetCS) PC shows the most negative ES1°* (−2.07 V vs SCE), more LE character (Stokes shift = 124 nm), and has an oxidizing PC radical cation (E1/2 = 0.30 V vs SCE). The CS PCs with improved properties, including more negative ES1°*, perform best in O-ATRP of MMA with the HetCS PC showing the best control in both DMAc (Đ = 1.08, I* = 89%) and EtOAc (Đ = 1.06, I* = 97%). Additionally, the HetCS PC was found to mediate the controlled polymerization of n-butyl acrylate (n-BA) (Đ = 1.24, I* = 97%), which has remained challenging in O-ATRP without supplemental deactivation strategies. An aryl CS PC was found to have moderate control as low as 1 ppm PC, indicating facilitation of low PC loadings (Đ = 1.33, I* = 69%). The relationship between excited state character, ES1°*, and polymerization control observed in this work provides a foundation for increasing the utility of phenazine PCs across photoredox catalysis.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


