Combined insights from DFT and microkinetics into NO reduction by CO over an LaFeO3 perovskite
IF 3.5
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
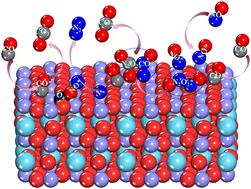
Herein, the relationship between the selective catalytic activity of CO in the reduction of NO and the active site of LaFeO3 perovskite was established through the combination of density functional theory and microkinetic studies. A reaction network consisting of various possible elementary reactions was built to reveal the pathway of CO2, N2 and N2O formation during CO-SCR on LaFeO3. The results indicated that the Fe site was active for reactant adsorption, which followed a chemisorption mechanism. The intermediate N2O2-mediated path was dominant for CO-SCR. Firstly, N2O2* was produced via the bimolecular reaction of NO-coupling with an energy barrier of 0.26 eV. Subsequently, N2O2* easily reacted with the adsorbed CO molecules to form an N2O2CO* intermediate (N2O2* + CO* → N2O2CO* + *), which required an activation energy of 0.65 eV. Finally, the formed N2O2CO* intermediate was reduced by CO* to generate N2 and CO2 (N2O2CO* + CO* → 2CO2 + N2 + 2*) with an energy barrier of 1.22 eV. Besides, the formation and decomposition of N2O were considered. N2O might have been formed via N–NO disproportionation reaction (NO* + N* → N2O + 2*) and decomposition of N2O2CO* (N2O2CO* → N2O + CO2 + *). Microkinetic results indicated that the conversion rate of CO and NO and the temperature showed a volcanic curve, and the N2 selectivity reached 100% at temperatures between 200 and 420 K. Thus, this work provides a detailed description of the CO-SCR reaction mechanism and lays the foundation for the development of high-performance LaFeO3 catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Dalton Transactions
化学-无机化学与核化学
CiteScore
6.60
自引率
7.50%
发文量
1832
审稿时长
1.5 months
期刊介绍:
Dalton Transactions is a journal for all areas of inorganic chemistry, which encompasses the organometallic, bioinorganic and materials chemistry of the elements, with applications including synthesis, catalysis, energy conversion/storage, electrical devices and medicine. Dalton Transactions welcomes high-quality, original submissions in all of these areas and more, where the advancement of knowledge in inorganic chemistry is significant.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


