Adapting systems biology to address the complexity of human disease in the single-cell era
IF 52
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
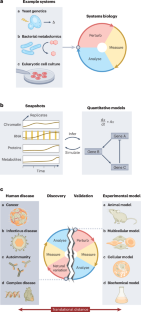
Systems biology aims to achieve holistic insights into the molecular workings of cellular systems through iterative loops of measurement, analysis and perturbation. This framework has had remarkable success in unicellular model organisms, and recent experimental and computational advances — from single-cell and spatial profiling to CRISPR genome editing and machine learning — have raised the exciting possibility of leveraging such strategies to prevent, diagnose and treat human diseases. However, adapting systems-inspired approaches to dissect human disease complexity is challenging, given that discrepancies between the biological features of human tissues and the experimental models typically used to probe function (which we term ‘translational distance’) can confound insight. Here we review how samples, measurements and analyses can be contextualized within overall multiscale human disease processes to mitigate data and representation gaps. We then examine ways to bridge the translational distance between systems-inspired human discovery loops and model system validation loops to empower precision interventions in the era of single-cell genomics. Differences between humans and experimental models create a translational gap that makes it difficult to extrapolate research findings. The authors review systems-focused approaches to identify and control the translational distance between a complex disease process being studied and the experimental model used for testing.

适应系统生物学,以解决单细胞时代人类疾病的复杂性
系统生物学旨在通过测量、分析和扰动的迭代循环,实现对细胞系统分子运作的整体洞察。这个框架在单细胞模式生物中取得了显著的成功,最近的实验和计算进展——从单细胞和空间分析到CRISPR基因组编辑和机器学习——提高了利用这种策略来预防、诊断和治疗人类疾病的令人兴奋的可能性。然而,适应系统启发的方法来解剖人类疾病的复杂性是具有挑战性的,因为人体组织的生物学特征与通常用于探测功能的实验模型之间的差异(我们称之为“翻译距离”)可能会混淆洞察力。在这里,我们回顾了样本、测量和分析如何在整体多尺度人类疾病过程中进行背景化,以减轻数据和代表性差距。然后,我们研究如何弥合系统启发的人类发现循环和模型系统验证循环之间的翻译距离,以增强单细胞基因组学时代的精确干预。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Nature Reviews Genetics
生物-遗传学
CiteScore
57.40
自引率
0.50%
发文量
113
审稿时长
6-12 weeks
期刊介绍:
At Nature Reviews Genetics, our goal is to be the leading source of reviews and commentaries for the scientific communities we serve. We are dedicated to publishing authoritative articles that are easily accessible to our readers. We believe in enhancing our articles with clear and understandable figures, tables, and other display items. Our aim is to provide an unparalleled service to authors, referees, and readers, and we are committed to maximizing the usefulness and impact of each article we publish.
Within our journal, we publish a range of content including Research Highlights, Comments, Reviews, and Perspectives that are relevant to geneticists and genomicists. With our broad scope, we ensure that the articles we publish reach the widest possible audience.
As part of the Nature Reviews portfolio of journals, we strive to uphold the high standards and reputation associated with this esteemed collection of publications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


