Genome-scale resources in the infant gut symbiont Bifidobacterium breve reveal genetic determinants of colonization and host-microbe interactions
IF 45.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Bifidobacteria represent a dominant constituent of human gut microbiomes during infancy, influencing nutrition, immune development, and resistance to infection. Despite interest in bifidobacteria as a live biotic therapy, our understanding of colonization, host-microbe interactions, and the health-promoting effects of bifidobacteria is limited. To address these major knowledge gaps, we used a large-scale genetic approach to create a mutant fitness compendium in Bifidobacterium breve. First, we generated a high-density randomly barcoded transposon insertion pool and used it to determine fitness requirements during colonization of germ-free mice and chickens with multiple diets and in response to hundreds of in vitro perturbations. Second, to enable mechanistic investigation, we constructed an ordered collection of insertion strains covering 1,462 genes. We leveraged these tools to reveal community- and diet-specific requirements for colonization and to connect the production of immunomodulatory molecules to growth benefits. These resources will catalyze future investigations of this important beneficial microbe.
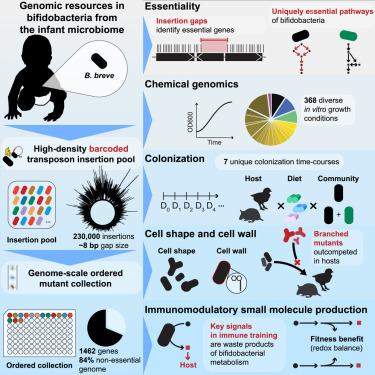
双歧杆菌是婴儿期人类肠道微生物组的主要成分,影响着营养、免疫发育和抗感染能力。尽管人们对双歧杆菌作为一种活的生物疗法很感兴趣,但我们对双歧杆菌的定植、宿主与微生物之间的相互作用以及双歧杆菌对健康的促进作用的了解还很有限。为了填补这些主要的知识空白,我们采用大规模遗传学方法创建了一个前列双歧杆菌突变适应性汇编。首先,我们生成了一个高密度随机条形码转座子插入池,并用它来确定无菌小鼠和鸡在多种饮食中定植期间的适应性要求,以及对数百种体外扰动的响应。其次,为了进行机理研究,我们构建了一个涵盖 1,462 个基因的有序插入菌株集合。我们利用这些工具揭示了群落和饮食对定植的特定要求,并将免疫调节分子的产生与生长益处联系起来。这些资源将促进未来对这种重要有益微生物的研究。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell
生物-生化与分子生物学
CiteScore
110.00
自引率
0.80%
发文量
396
审稿时长
2 months
期刊介绍:
Cells is an international, peer-reviewed, open access journal that focuses on cell biology, molecular biology, and biophysics. It is affiliated with several societies, including the Spanish Society for Biochemistry and Molecular Biology (SEBBM), Nordic Autophagy Society (NAS), Spanish Society of Hematology and Hemotherapy (SEHH), and Society for Regenerative Medicine (Russian Federation) (RPO).
The journal publishes research findings of significant importance in various areas of experimental biology, such as cell biology, molecular biology, neuroscience, immunology, virology, microbiology, cancer, human genetics, systems biology, signaling, and disease mechanisms and therapeutics. The primary criterion for considering papers is whether the results contribute to significant conceptual advances or raise thought-provoking questions and hypotheses related to interesting and important biological inquiries.
In addition to primary research articles presented in four formats, Cells also features review and opinion articles in its "leading edge" section, discussing recent research advancements and topics of interest to its wide readership.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


