Indocyanine polymethine fluorophores with extended π-conjugation emitting beyond 1,200 nm for enhanced NIR-II imaging
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
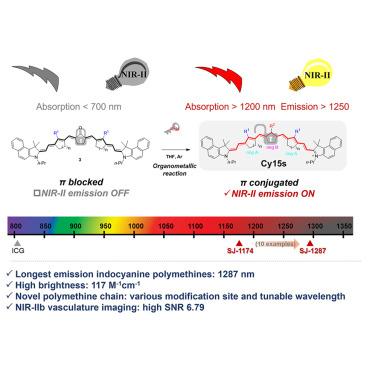
Indocyanine polymethines are among the most clinically promising probes for fluorescence imaging. Longer wavelength NIR-II probes offer enhanced fluorescence imaging performance by improving the tissue penetration depth and signal-to-noise ratio (SNR), but this often results in reduced brightness. Despite multiple attempts to redshift indocyanine polymethines' wavelengths, their emission wavelengths are restricted to 1,103 nm. We report the first indocyanine polymethines, Cy15s, emitting beyond 1,200 nm, with the longest maximum peak emission at 1,287 nm while maintaining a high brightness of 117.1 M−1⋅cm−1 in dichloroethane (DCM), 6-fold of the best performance of polymethine fluorophores emitting over 1,200 nm. The low cytotoxicity and remarkable optical properties enable high-quality near-infrared II (NIR-II)b angiography and long-term orthotopic tumor imaging. In addition to the relatively mature terminal groups research of polymethines, this study introduces a novel scaffold for conjugation chains, opening new avenues for the design and synthesis of NIR-II probes for deep-tissue imaging and tumor research.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


