Monoselective Histone Deacetylase 6 PROTAC Degrader Shows In Vivo Tractability
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
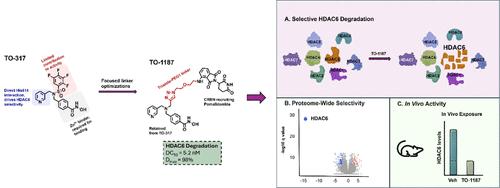
Herein, we report a potent HDAC6 PROTAC, TO-1187, which selectively degrades HDAC6 in cellulo and demonstrates in vivo efficacy. The design of TO-1187 was achieved by linking our previously reported HDAC6 inhibitor, TO-317, to the cereblon (CRBN) E3 ligase ligand, pomalidomide. TO-1187 achieved monoselective HDAC6 degradation in human multiple myeloma cells, MM.1S, with a Dmax of 94% and a DC50 of 5.81 nM after 6 h. Importantly, at concentrations up to 25 μM, TO-1187 exhibited no cellular degradation of other HDACs. Proteomic evaluation confirmed a highly selective proteome-wide degradation profile, with HDAC6 the only protein observed to be depleted. Notably, TO-1187 did not impact the abundance of well-known CRBN neosubstrates, like IKZF1, IKZF3, CK1α, SALL4, and GSPT1. In vivo evaluation confirmed that TO-1187 efficiently degraded HDAC6 in mouse tissues, measured 6 h after intravenous injection. In summary, TO-1187 represents a viable candidate for advanced preclinical evaluation of HDAC6 biology.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


