Ni(ii)/spiroBox-catalyzed asymmetric Friedel–Crafts alkylation of indoles with nitroalkenes†
IF 4.6
3区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Nickel complexes of chiral spiroBox ligand catalyzed Friedel–Crafts alkylation reaction of indoles with nitroalkenes. Excellent yields (up to 99%) and enantiomeric excess (ee) values (up to 97%) were obtained with a broad scope of substrates. This catalytic system provides a facile synthesis of optically active 2-indolyl-1-nitro derivatives with high yield and enantioselectivity.
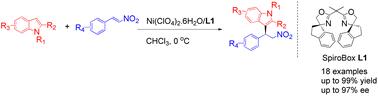
Ni(ii)/ spirobox催化吲哚与硝基烯的不对称Friedel-Crafts烷基化反应
手性spiroBox配体镍配合物催化吲哚与硝基烯的Friedel-Crafts烷基化反应。在广泛的底物范围内获得了优异的产率(高达99%)和对映体过量(ee)值(高达97%)。该催化体系可方便地合成具有高收率和对映选择性的光学活性的2-吲哚-1-硝基衍生物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

RSC Advances
chemical sciences-
CiteScore
7.50
自引率
2.60%
发文量
3116
审稿时长
1.6 months
期刊介绍:
An international, peer-reviewed journal covering all of the chemical sciences, including multidisciplinary and emerging areas. RSC Advances is a gold open access journal allowing researchers free access to research articles, and offering an affordable open access publishing option for authors around the world.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


