Exosomal delivery of rapamycin modulates blood-brain barrier penetration and VEGF axis in glioblastoma
IF 10.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
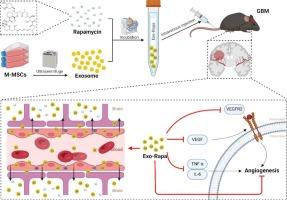
Exosomes (Exos), nanosized membranous vesicles (30–160 nm), have been validated as an effective drug delivery system capable of traversing biological barriers. Mesenchymal stem cells (MSCs), due to their near-limitless self-renewal capabilities, provide a plentiful source of exosomes for clinical applications. In this study, we utilized an exosome-encapsulated rapamycin (Exo-Rapa) delivery strategy, which permits the use of smaller drug dosages to achieve effects typically seen with higher dosages, thus enhancing drug efficacy. Moreover, Exos can transport pharmaceuticals across the blood-brain barrier (BBB) to the brain, and further penetrate GL261 cells to exert their effects. Within the tumor microenvironment, Exo-Rapa is released more rapidly and efficiently at the tumor site. The acidic conditions in tumors accelerate the release of Exo-Rapa, a characteristic that may make it a promising targeted therapeutic in future cancer research. Additionally, a series of in vivo experiments have further demonstrated the permeability of Exo-Rapa across the BBB, enabling it to accumulate at tumor sites; it also ameliorates inflammatory responses in Glioblastoma multiforme (GBM) mouse models and enhances anti-tumor activity through the regulation of angiogenesis via the VEGF/VEGFRs axis. Our results indicate that MSC-derived exosomes are a potent therapeutic carrier for GBM, offering an effective strategy for enhancing drug delivery across the BBB and providing a scientific foundation for the use of exosomes in the treatment of GBM and other diseases.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


