Dissociation-dependent kinetics and distinct pathways for direct photolysis and OH/SO4− radical dominated photodegradation of ionizable antiviral drugs in aquatic systems
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
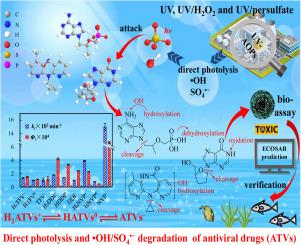
Advanced oxidation processes (AOPs), such as UV, UV/H2O2 and UV/persulfate, are widely used to remove emerging organic contaminants from wastewater streams. However, knowledge on chemical degradation pathways, reaction kinetics as well as formation and toxicity of key degradates is limited. We investigated the direct photolysis and •OH/SO4•− dominated kinetics, intermediates and toxicity evolution of three ionizable antiviral drugs (ATVs): tenofovir (TFV), didanosine (DDI), and nevirapine (NVP). Their transformation kinetics were found to depend on the dominant protonated states. Under UV-Vis irradiation (λ > 290 nm), TFV and DDI photolyzed the fastest in the cationic forms (H2TFV+ and H2DDI+), whereas NVP exhibited the fastest photodegradation in the anionic forms (NVP−). The anionic forms (TFV− and NVP−) demonstrated the highest reactivities towards •OH in most cases, while the cationic forms (H2DDI+ and H2NVP+) reacted the fastest with SO4•− for most of the ATVs. The dissociation-dependent kinetics can be attributed to the discrepancies in deprotonation degrees, quantum yields, electron densities and coulombic repulsion with SO4•− in their dissociated forms. Based on the key product identification via HPLC-MS/MS, the pathways involved hydroxylation, dehydroxylation, oxidation, reduction, cyclopropyl cleavage, C-N breaking, elimination, cyclization and deamidation reactions, which can be prioritized based on the specific compound and the photochemical process. Furthermore, a bioassay showed the photomodified toxicity of the ATVs to Vibrio fischeri (bioluminescent bacteria) during the three processes, which was also demonstrated by ECOSAR model assessment. Nearly half of the chemical intermediates were demonstrably more toxic than their respective parent ATVs. These results provide new insights into understanding the persistence, fate and hazards associated with applying the UV-assisted AOPs to treat wastewater containing ATVs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


