Tunable Multicolor Luminescence in Double Perovskite [(CH3)4N]2KEu1–xTbx(NO3)6 Single Crystals
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
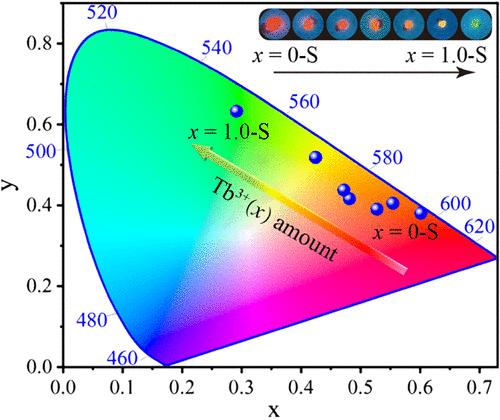
Tunable multicolor luminescence materials can flexibly meet the needs of smart lighting, enabling efficient light energy use and minimizing waste. Lead-free hybrid double perovskites A2MIMIII(NO3)6 hold great potential in luminescence, benefiting from their tunable composition, high light absorption, low synthesis cost, and environmental friendliness. However, achieving tunable multicolor emission within a single matrix of these materials has yet to be realized. In this study, a series of [(CH3)4N]2KEu1–xTbx(NO3)6 single crystals have been synthesized using an environmentally friendly and mild aqueous solution evaporation method. The two emitter centers, Tb3+ and Eu3+, display energy transfer from Tb3+ to Eu3+. The emission color of the as-synthesized crystals gradually changes from red to orange, then to yellow, and finally to green with an increase of Tb3+ concentration, achieving yellow and green light emission in the three-dimensional rare-earth hybrid double perovskites for the first time. Green emission from [(CH3)4N]2KTb(NO3)6 displays the highest quantum yield at 87%. The millisecond-level emission decay time and high decomposition temperatures (365 °C) of [(CH3)4N]2KEu1–xTbx(NO3)6 single crystals highlight their potential for use in luminescent devices and phosphors, among other fields.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


