An Oxidative Stress Nanoamplifier with Efficient Non-Fenton-Type Hydroxyl Radical Generation and Sulfur Dioxide Release for Synergistic Treatment of Tumor
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
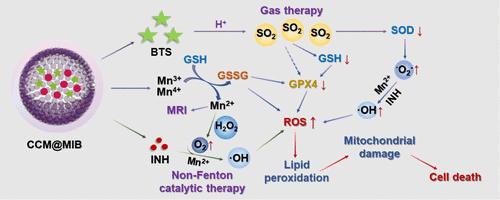
Overcoming tumor antioxidant defenses remains a critical challenge for reactive-oxygen-species-mediated tumor therapies. To address this problem, herein, a theranostic nanomedicine designated as CCM@MIB has been elaborately constructed. Homologous cancer cell membrane (CCM) camouflage significantly enhances the selective accumulation of the nanomedicine at tumor sites. In response to the tumor microenvironment (TME), CCM@MIB controllably releases Mn ions and sulfur dioxide (SO2) molecules. The released Mn ions catalyze the self-oxidation of isoniazid to generate highly toxic •OH, while the SO2 produced by benzothiazole sulfinate effectively disrupts tumor antioxidant defense systems. The catalase-like activity endowed by Mn ions and the increased intracellular •O2– level induced by SO2 further promote •OH production. Therefore, such an intellectual combination of non-Fenton-type catalytic therapy and SO2 gas therapy significantly amplifies oxidative stress and efficiently suppresses tumor growth. Additionally, the TME-activated magnetic resonance imaging contrast performance of CCM@MIB is beneficial for guiding antitumor treatment. This considerate strategy designed in our work provides an ingenious paradigm for the development of efficient antitumor therapies.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


