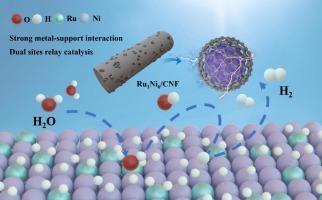
Engineering Ru and Ni sites relay catalysis and strong metal-support interaction for synergetic enhanced electrocatalytic hydrogen evolution performance
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Electrocatalytic hydrogen evolution is the crucial technology of renewable energy conversion and fuel production in the future energy system. However, its catalytic performance is often limited by multiple step requirements of alkaline HER and trade-off between the activity and stability. Water dissociation, as a fundamental step in alkaline HER, directly impacts the overall efficiency of electrocatalytic hydrogen evolution. Here, the Ru-doped Ni nanoparticles coated with graphite carbon shell in carbon nanofiber (Ru1Ni6/CNF) was constructed by electrospinning and pyrolysis process. By changing the ratio of Ru to Ni, the synergistic effect between Ru site and Ni site was amplified, and the relay synergistic reaction between Ru site and Ni site was realized. Strong metal-support interaction (SMSI) formed between Ru-doped Ni nanoparticles and graphite carbon shell not only protects the catalyst from the loss of specific surface area caused by thermal agglomeration, but also promotes charge transfer. Graphite carbon shell can protect the catalyst surface from (electro-) chemical corrosion. The Ru1Ni6/CNF exhibits a low overpotential of 14 mV at 10 mA cm−2 and shows excellent stability over 400 h in 1 M KOH solution. DFT shows that the Ru site has high adsorption and desorption efficiency for water, which is essential for the water dissociation step in alkaline HER, while Ni site promotes hydrogen recombination. The performance of the dual sites relay catalyst is comparable to that of the commercial precious metal catalyst. This study provides a prospect for the design of multi-sites and multifunctional component cooperative reaction catalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


