Azacitidine-induced bullous pemphigoid-like localized toxic reaction.
IF 0.9
Q2 MEDICINE, GENERAL & INTERNAL
Einstein-Sao Paulo
Pub Date : 2025-03-03
eCollection Date: 2025-01-01
DOI:10.31744/einstein_journal/2025RC0699
引用次数: 0
Abstract
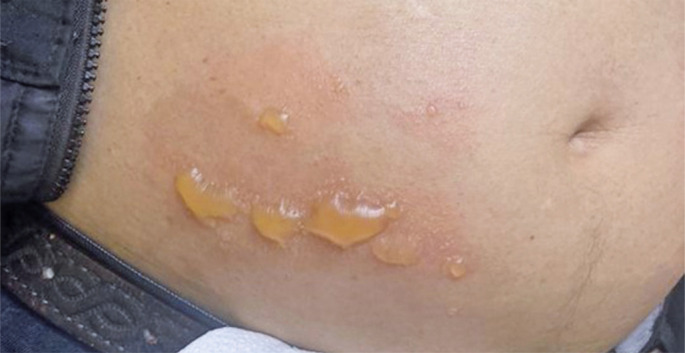
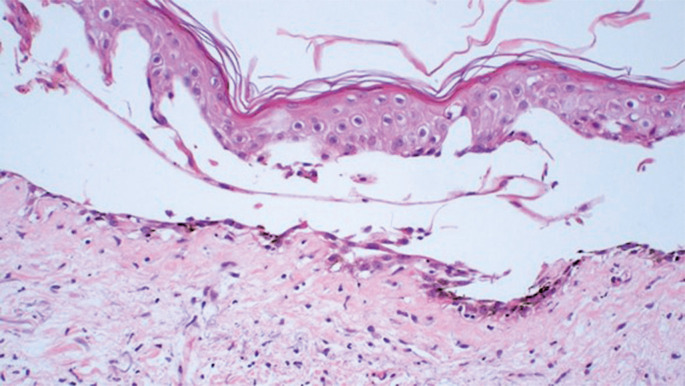
Azacitidine is a hypomethylating agent recommended for the treatment of patients with high-risk myelodysplastic syndromes. Here, we report the case of a patient with myelodysplastic syndrome who was not eligible for allogeneic stem cell transplantation (allo-SCT) and presented with a rare and previously unreported cutaneous side effect after the use of subcutaneous azacitidine. We propose that changing the route of azacitidine administration from subcutaneous to intravenous could potentially decrease the occurrence of bullous pemphigoid-like localized toxic reactions in some patients.
阿扎胞苷诱导的大疱性类天疱疮样局部毒性反应。
阿扎胞苷是一种低甲基化药物,推荐用于治疗高危骨髓增生异常综合征患者。在这里,我们报告了一例骨髓增生异常综合征患者,他不符合同种异体干细胞移植(alloc - sct)的条件,并且在使用皮下阿扎胞苷后出现了罕见且以前未报道的皮肤副作用。我们建议将阿扎胞苷的给药途径从皮下改为静脉注射,可能会减少一些患者大疱性类天疱疮样局部毒性反应的发生。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Einstein-Sao Paulo
MEDICINE, GENERAL & INTERNAL-
CiteScore
2.00
自引率
0.00%
发文量
210
审稿时长
38 weeks
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


