Best Practices for Variable-Temperature Electrochemistry Experiments and Data Reporting
IF 19.3
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
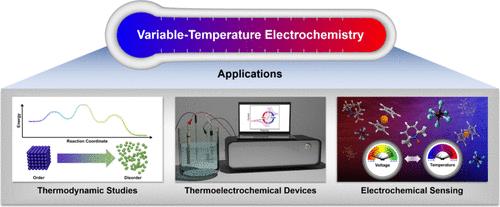
Figure 1. Overview of select applications of variable-temperature electrochemistry. Thermal and Solution Stability. Identify the temperature range and solvent(s) in which the redox-active analyte of interest is chemically and electrochemically stable. Such evaluations may for example be performed using NMR spectroscopy or UV–visible absorption spectroscopy in conjunction with cyclic voltammetry at varying temperatures in different solvents. Electrochemical Stability. Assess the stability of different charge states of the redox-active analyte of interest through controlled potential electrolysis experiments over extended periods of time. Such experiments may for example help in identifying phase-change reactions involving precipitation. Kinetic Stability. Identify the kinetics of chemical and electrochemical reactions associated with the redox system of interest. Assess if the redox-active analyte is resistant to chemical and electrochemical changes over the time course of the electrochemical experiment. Time-dependent electrochemical measurements, including variable-scan-rate cyclic voltammetry and controlled potential/current vs time experiments may inform kinetic stability in the relevant time scale. For instance, the intensity ratios of the anodic and cathodic peak currents in the voltammograms collected at variable scan rates can inform the rate of electron-transfer reactions and help determine any operable kinetic constraints. Chemical Equilibria. Identify the chemical equilibria that apply to the redox system of interest. Assess whether homogeneous liquid-phase reactions or multiphase processes are involved, and determine if these reactions are sensitive to the solution proton activity. Standard analytical techniques used to study liquids, gases, and solids may be employed for such assessments. Electrochemical measurements in solutions of variable proton activity can shed light on the sensitivity of the given electron-transfer reactions toward protons, as is the case for proton-coupled electron-transfer reactions. Influence of other ions, such as those of the supporting electrolyte, may be investigated in a similar manner. Figure 2. Schematic representations of variable-temperature open circuit potential (a) and cyclic voltammetry (b) data (left) and corresponding plots of open circuit potential or half-wave potential vs temperature obtained under isothermal conditions (right). Note that for analogous nonisothermal measurements, the x-axis in the plots on the right would be temperature difference instead of temperature. Figure 3. Schematic representations of experimental setups used for variable-temperature electrochemical studies: isothermal (a) and nonisothermal (b) setups. WE, CE, and RE denote working electrode, counter electrode, and reference electrode, respectively. The additional black-capped metal rods immersed in the solutions denote temperature probes. Figure 4. Schematic representations of electrochemical cell configurations used for different types of isothermal (a, b) and nonisothermal (c, d) variable-temperature electrochemical measurements. WE, CE, and RE denote working electrode, counter electrode, and reference electrode, respectively. The additional black-capped metal rods immersed in the solutions denote temperature probes. Connections between glass compartments in panels b–d feature a fine-porosity glass frit. Identify the Properties of the Desired Redox System. As a first step, employ analytical and electroanalytical tools to decipher the chemical and electrochemical properties of the redox system of interest. This includes assessment of chemical equilibria, thermal and solution stability, electrochemical stability, and kinetic stability. The choice of method to quantify temperature coefficients will be significantly impacted by the characteristics of the given redox system. Carefully Consider Experimental Protocols. Provide a detailed experimental section describing the conditions used for electrochemical measurements, including the type of measurements, compositions of solutions, experimental setup, and what (if any) corrections are made to the data. Be aware of experimental conditions where additional corrections, such as for thermodiffusion and thermal liquid junction potential, should be made. Use the same experimental conditions (solvent, supporting electrolyte, measuring mode, etc.) when comparing temperature coefficients of different redox-active analytes. Record Temperature with Accuracy. Use an internal high-resolution digital thermometer to monitor the temperature in the vicinity of the electrodes to ensure accurate and reliable temperature measurements. The thermometers should be placed close to the electrodes in solutions and calibrated periodically using an external temperature controller. Provide Appropriate Quantification of Temperature Coefficients. Use VT-OCP rather than VT-CV measurements for electrochemical systems in which both the oxidized and reduced forms of the redox-active analyte can be chemically isolated. The VT-OCP method can also yield accurate temperature coefficients in cases where sluggish kinetics of electron-transfer reactions may be a concern. Carry out VT-CV measurements under isothermal conditions only, as nonisothermal VT-CV measurements are typically less reliable. Correct all variable-temperature electrochemical data collected under isothermal conditions for the temperature coefficient of the reference electrode potential, determined using the same reference electrode and supporting electrolyte solution as in the presence of the redox-active analyte. For systems that are sensitive to solution proton activity, report temperature coefficients for specific pH ranges. The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsenergylett.5c00308. Experimental details, additional electrochemical data, and overview of select literature reports on variable-temperature electrochemical studies of solution-phase systems (PDF) Most electronic Supporting Information files are available without a subscription to ACS Web Editions. Such files may be downloaded by article for research use (if there is a public use license linked to the relevant article, that license may permit other uses). Permission may be obtained from ACS for other uses through requests via the RightsLink permission system: http://pubs.acs.org/page/copyright/permissions.html. A.D., B.K., J.K., and A.E.T. acknowledge support from the University of Rochester. M.D. acknowledges support from the National Science Foundation (NSF) through Award CBET-2350223. This work made use of the CENTC Elemental Analysis Facility at the University of Rochester, funded by the NSF through Award CHE-0650456 and a JEOL NMR spectrometer acquired with support from the NSF through MRI Award CHE-2215973. This article references 48 other publications. This article has not yet been cited by other publications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Energy Letters
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
31.20
自引率
5.00%
发文量
469
审稿时长
1 months
期刊介绍:
ACS Energy Letters is a monthly journal that publishes papers reporting new scientific advances in energy research. The journal focuses on topics that are of interest to scientists working in the fundamental and applied sciences. Rapid publication is a central criterion for acceptance, and the journal is known for its quick publication times, with an average of 4-6 weeks from submission to web publication in As Soon As Publishable format.
ACS Energy Letters is ranked as the number one journal in the Web of Science Electrochemistry category. It also ranks within the top 10 journals for Physical Chemistry, Energy & Fuels, and Nanoscience & Nanotechnology.
The journal offers several types of articles, including Letters, Energy Express, Perspectives, Reviews, Editorials, Viewpoints and Energy Focus. Additionally, authors have the option to submit videos that summarize or support the information presented in a Perspective or Review article, which can be highlighted on the journal's website. ACS Energy Letters is abstracted and indexed in Chemical Abstracts Service/SciFinder, EBSCO-summon, PubMed, Web of Science, Scopus and Portico.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


