Rational Design of Near-Infrared Reflective Pigments by Adjusting the Ni2+ Coordination Environment
IF 4.3
2区 化学
Q1 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
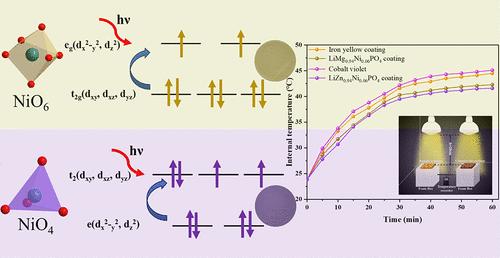
Inorganic pigments offer excellent potential because of their resilience in a variety of environments, and high-reflectance near-infrared (NIR) pigments are a useful tactic to reduce the heat island effect. Nonetheless, the design of inorganic pigments is still dominated by serendipity, and it has never been easy to come up with logical designs for NIR reflective inorganic pigments. By modifying the coordination environment of Ni2+ using the generic formulas LiMg1–xNixPO4 and LiZn1–xNixPO4 (x = 0.02–0.12), yellow and violet pigments were successfully developed in this research. According to structural investigations, Ni2+ effectively replaced Mg2+ and Zn2+ to create [NiO4] tetrahedra and [NiO6] octahedra, causing an orderly alteration of the cell parameters. The diverse transition modes of Ni2+ in various crystal fields are the cause of the noticeable color changes caused by the solid solutions, according to the UV–vis-NIR spectra. Furthermore, both pigments have outstanding NIR solar reflectivity and coloring capabilities. Both the sample-colored coatings and the coating’s surface under infrared light demonstrated lower temperatures than the commercial pigmented coatings for thermal insulation applications.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganic Chemistry
化学-无机化学与核化学
CiteScore
7.60
自引率
13.00%
发文量
1960
审稿时长
1.9 months
期刊介绍:
Inorganic Chemistry publishes fundamental studies in all phases of inorganic chemistry. Coverage includes experimental and theoretical reports on quantitative studies of structure and thermodynamics, kinetics, mechanisms of inorganic reactions, bioinorganic chemistry, and relevant aspects of organometallic chemistry, solid-state phenomena, and chemical bonding theory. Emphasis is placed on the synthesis, structure, thermodynamics, reactivity, spectroscopy, and bonding properties of significant new and known compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


