Discovery of New Azaindole Metallo-Deubiquitinase CSN5 Inhibitors
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
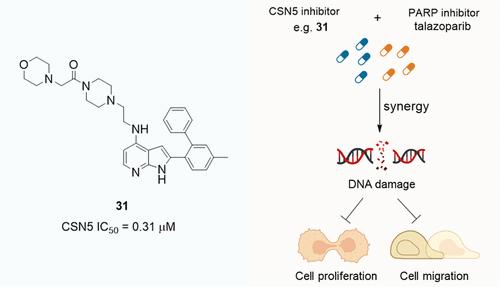
CSN5 is responsible for the deneddylation of cullin-RING E3 ubiquitin ligases and is closely linked to the development of various cancers. We previously developed a noncatalytic activity assay platform using novel fluorescent probes derived from azaindole inhibitors, which also highlighted the potential for further structural optimization of azaindoles. Herein, we report a series of new 4-NH-substituted azaindole derivatives, some of which showed nanomolar activity against the CSN5 subunit. Cellular assays revealed that the new azaindoles increase the cullin 1 neddylation in cancer cells. Importantly, they exhibit synergistic anticancer effects in combination with poly(ADP-ribose) polymerase inhibitors through increasing DNA damage. This work presents a new lead compound and a potential combination strategy for drug discovery targeting CSN5.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


