Electrodeposited P-Doped CuNi Alloy from Deep Eutectic Solvent for Efficient and Selective Nitrate-to-Ammonia Electroreduction
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
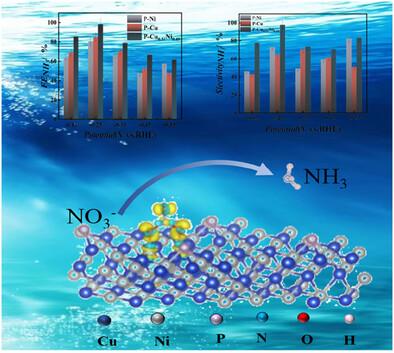
Electrochemical nitrate reduction reaction (NO3RR) offers a promising alternative for ammonia production using electricity generated from renewable energy sources. Active electrocatalysts with high selectivity and high yield are required to selectively catalyze NO3RR to ammonia. Here, P-doped Cu0.51Ni0.49 alloy thin films are electrodeposited from a deep eutectic solvent of choline chloride-ethylene glycol (ChCl/EG). The P-Cu0.51Ni0.49 produces 1616.94 µg h−1 cm−2 of ammonia at −0.55 VRHE (V versus reversible hydrogen electrode), with a Faradaic efficiency of 98.38% and ammonia selectivity of 97.84% at −0.25 VRHE, much better than the P-Ni and P-Cu prepared under similar condition. The high ammonia production rate, Faradaic efficiency and selectivity are originated from high number of electrochemically active sites and more facile kinetics. Mechanistic study and density functional theory calculation proves that P-Cu0.51Ni0.49 exhibits higher conductivity and more facile NO3− adsorption compared to P-Ni and P-Cu, induced by the electron interaction. Characterizations after NO3RR cycling show that the crystallinity of P-Cu0.51Ni0.49 decreases, with the content of divalent metal ions increases at the surface. The P-Cu0.51Ni0.49 is an active and stable material to electrocatalyze NO3RR to ammonia in neutral aqueous solutions.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


