Boost photoelectrocatalytic selectivity of glycerol to dihydroxyacetone on BiVO4 via accelerating middle hydroxyl oxidation by co-catalysts
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
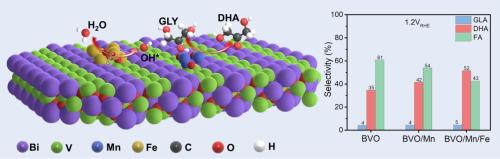
Enhancing the selective oxidation of glycerol into 1,3-dihydroxyacetone (DHA) on a photoanode is attractive but challenging due to the complex reaction pathway. At present, the 20 % selectivity of DHA products on a BiVO4 photoanode in neutral condition as well as its photocorrosion prove to be below expectations. In this work, we decorated binary co-catalysts MnOx/FeOOH on BiVO4 (denoted BVO/Mn/Fe) for the selective photoelectrocatalytic glycerol to DHA. The BVO/Mn/Fe photoanode exhibited a DHA selectivity of 52 % at 1.2 VRHE with an evolution rate of 193.3 mmol m-2h−1 in pH = 7.0, and maintained 97.2 % of the initial photocurrent after 12 h stability test. The role of MnOx and FeOOH in the selective oxidation of glycerol was investigated through comprehensive spectroscopic and computational methods. Fourier transform infrared spectroscopy showed that the middle hydroxyl group preferentially adsorbs onto MnOx surfaces, density function theory calculations verified the optimized rate-determining step and decreased energy barrier by MnOx. Meanwhile, FeOOH significantly increased the active surface states on BiVO4 and produced more hydroxyl radicals. The synergistic effect of MnOx and FeOOH promoted the middle hydroxyl oxidation of glycerol and conversion efficiency to DHA on BiVO4 under neutral condition.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


