Synthesis and preclinical evaluation of an Al18F radio-fluorinated bivalent PD-L1 nanobody
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
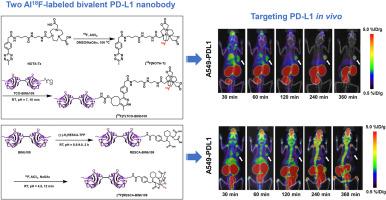
Immunotherapy targeting the programmed death 1/programmed death ligand 1 (PD-1/PD-L1) pathway has achieved remarkable clinical success, but there is a shortage of effective approaches for screening suitable patients. Recently developed PD-L1 nanobody probes have limitations, including limited availability of radionuclides, short tumor retention times, and accumulation in non-target organs. To enhance tumor retention and improve tumor-to-normal tissue contrast, we herein report the synthesis and preclinical evaluation of two Al18F-labeled bivalent PD-L1 nanobody probes ([18F]TzTCO-BINb109 and [18F]RESCA-BINb109). Preliminary results indicated that [18F]TzTCO-BINb109 had a greater affinity for PD-L1 and better stability than [18F]RESCA-BINb109. Micro-PET/CT revealed that [18F]TzTCO-BINb109 uptake in A549-PDL1 tumors peaked at 240 min post-injection (3.19 ± 0.49 %ID/g) and demonstrated sustained retention without in vivo defluorination. In contrast, [18F]RESCA-BINb109 exhibited shorter tumor retention (at 60 and 240 min, 2.08 ± 0.22 and 1.37 ± 0.26 %ID/g, respectively) and significant defluorination in vivo. Ex vivo biodistribution studies revealed that the tumor uptake of [18F]TzTCO-BINb109 was consistent with the PET results, with the highest uptake by A549-PDL1 tumor cells (3.43 ± 0.94 %ID/g) compared with H1975 (0.93 ± 0.18 %ID/g) and A549 (0.68 ± 0.12 %ID/g) cells observed at 240 min post-injection. Compared with the previously reported monomeric PD-L1-targeting nanobody probe, [68Ga]NOTA-Nb109, [18F]TzTCO-BINb109 demonstrated enhanced tumor uptake, prolonged retention, and superior tumor-to-normal tissue contrast, contributing to higher imaging quality. These results confirmed that the bivalent PD-L1 nanobody radioligand, [18F]TzTCO-BINb109, was a promising diagnostic probe for PD-L1 detection, efficacy evaluation, and prescription optimization of immune checkpoint inhibitor therapies.

求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


