Noninnocent Spectator Ligands Facilitate CO Ligand-Stabilized Mn(I) Metal-Catalyzed Hydrogenation of Urea Derivatives or Carbamates to the More Reactive Formamides
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The carbonyl ligands that stabilize the Mn(I) center in the Mn(CO)5Br metal precursor are usually retained upon complexation with the tridentate ligand; however, their presence limits the hydrogenation performance of the Mn complex. Herein, we report the highly selective semihydrogenation of urea derivatives or carbamates, two of the most challenging carbonyl compounds, and polyurethanes to more active formamides using a catalyst system containing earth-abundant metal Mn under mild reaction conditions, which has been previously achieved using precious metal Ru or Ir catalysts. This catalytic activity stems from the fact that the Mn complex bears a noninnocent ligand, which facilitates the simultaneous transfer of both hydrogen atoms from a dihydrogen molecule, thereby avoiding the energetically demanding dihydrogen coordination step observed in systems with exclusively innocent ligands. Additionally, DFT calculations provide insights into the reason for the selective hydrogenation of urea or carbamates to more reactive formamides.
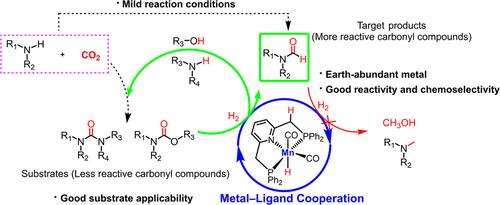
非无害旁观者配体促进CO配体稳定的Mn(I)金属催化尿素衍生物或氨基甲酸酯加氢成更活泼的甲酰胺
在Mn(CO)5Br金属前驱体中稳定Mn(I)中心的羰基配体通常在与三齿配体络合后保留;然而,它们的存在限制了锰配合物的加氢性能。在此,我们报道了尿素衍生物或氨基甲酸酯(两种最具挑战性的羰基化合物)和聚氨酯在温和的反应条件下使用含有丰富的金属Mn的催化剂体系进行高选择性半氢化反应,以获得更活跃的甲酰胺,这在以前是使用贵金属Ru或Ir催化剂实现的。这种催化活性源于Mn配合物带有一个非无害配体,这有助于同时从二氢分子转移两个氢原子,从而避免了在完全无害配体的系统中观察到的能量要求高的二氢配位步骤。此外,DFT计算提供了尿素或氨基甲酸酯选择性加氢生成反应性更强的甲酰胺的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


