Bicyclo[4.1.1]octanes via Strain-Storage Cyclobutanone-Alkyne Coupling and Its Enantioselective Strain-Release Transformation to Bicyclo[4.2.1]nonanes
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Three-dimensional C(sp3)-rich bicyclic scaffolds are vital saturated bioisosteres and versatile building blocks in medicinal and synthetic chemistry. Notwithstanding the importance and progress, the synthesis of bicyclo[4.1.1] and bicyclo[4.2.1] scaffolds remains challenging. Herein, we unveil a rare nickel-catalyzed strain-storage cyclobutanone-alkyne coupling to prepare various functionalized bicyclo[4.1.1]octanes. Moreover, downstream strain-release transformation of bicyclo[4.1.1] scaffolds via rhodium-catalyzed enantioselective sequential C–C activation and 1,4-rhodium shift was efficiently achieved to fuse a variety of enantioenriched bicyclo[4.2.1] scaffolds with high chemo-, diastereo-, and enantioselectivity. Mechanistic studies revealed the well-tailored spironickelabicyclic intermediate with a rigid endo-cyclic olefin favors the strain-storage cyclometalation over the common strain-release-driven β-carbon elimination.
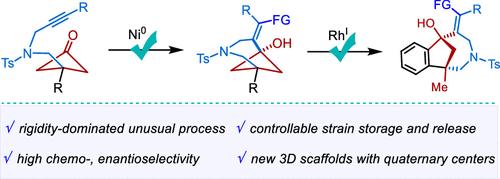
双环[4.1.1]辛烷通过菌株储存环丁酮-炔偶联及其对映选择性菌株释放转化为双环[4.2.1]壬烷
三维富含C(sp3)的双环支架是重要的饱和生物同位体,是药物和合成化学中多功能的基础材料。尽管其重要性和进展,但双环[4.1.1]和双环[4.2.1]支架的合成仍然具有挑战性。在此,我们揭示了一种罕见的镍催化的菌株存储环丁酮-炔偶联,以制备各种功能化的双环[4.1.1]辛烷。此外,通过铑催化的对映选择性顺序C-C活化和1,4-铑位移,有效地实现了双环[4.1.1]支架的下游菌株释放转化,融合了多种具有高化学选择性、非映选择性和对映选择性的富含对映体的双环[4.2.1]支架。机制研究表明,与普通的菌株释放驱动的β-碳消除相比,具有刚性内环烯烃的精心定制的螺环环中间体更有利于菌株储存环金属化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


