Metal-free porphyrin porous organic cage for efficient iodine capture
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
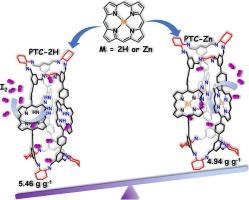
A large amount of radioactive iodine, which is harmful to the environment and humans, is produced during the operation of nuclear power plants, and the design of adsorbents for the efficient adsorption of radioactive iodine should be further studied. In this work, porphyrin-based porous organic cage (POC), denoted as PTC-2H, was synthesized using dynamic covalent chemistry, and PTC-Zn was obtained by the post-synthetic modification of PTC-2H with Zn2+. Subsequently, the iodine capture performance was studied. The iodine capture capacity of PTC-2H reached 5.46 g g−1, exceeding the capacities reported for most POCs. Multi-spectral techniques confirmed that iodine adsorption occurs mainly through chemical adsorption, and porphyrin units together with imine bonds are the main active sites for iodine adsorption. Owing to the synergistic effect of the high specific surface area, large conjugated plane, and abundant N heteroatoms, PTC-2H exhibited a higher iodine capacity than other POCs. After the introduction of zinc ions into PTC-2H, PTC-Zn exhibited a lower iodine capacity (4.96 g g−1), which could be attributed to the loss of the –NH bonds in the porphyrin units. Thus, POCs containing porphyrin units were used in this study for iodine adsorption, and the positive effects of –NH bonds in the N4 cavity of the porphyrin plane, together with the higher specific surface area of PTC-2H, on the iodine adsorption capacity are demonstrated proved for the first time. The insights obtained in this study are of great significance for exploring new materials with more functional groups for improving their iodine adsorption performance.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


