HuR prevents amyloid beta-induced phase separation of miRNA-bound Ago2 to RNA-processing bodies
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
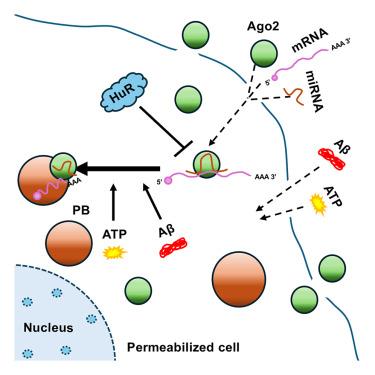
Phase separation into membrane-less organelles regulates protein activity in eukaryotic cells. miRNA-repressed mRNAs and Ago proteins localize to RNA-processing bodies (P-bodies), which are subcellular structures formed by several RNA-binding and regulatory proteins. Ago2, the essential miRNA-binding protein, forms a complex with miRNAs to repress protein synthesis by binding to mRNAs and targeting them to P-bodies. However, factors controlling Ago2 and miRNA-repressed mRNA compartmentalization into P-bodies are not fully understood. We developed a detergent-permeabilized cell-based assay system to observe the phase separation of exogenously added Ago2 into P-bodies in vitro. We observed that miRNA binding to Ago2 is essential for its localization to P-bodies, which is also ATP dependent. Osmolarity and salt concentration also affect Ago2 compartmentalization to P-bodies. Amyloid beta oligomers enhance Ago2 targeting to P-bodies by slowing down cellular Ago2 dynamics and inhibiting mTORC1 activity. However, the RNA-binder HuR disrupts P-body targeting by “sponging” out Ago2-associated miRNAs.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


