Hydroamination of terminal alkynes mediated by W(0) complex: A convenient tool for new enamines synthesis
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
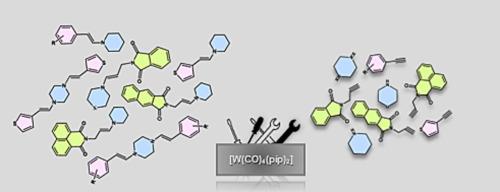
Herein, we report the efficient synthesis of E-enamines with 100% regioselectivity by the hydroamination of various terminal alkynes with secondary amines (piperidine and piperazine) mediated by easily accessible tungsten(0) complex, at smooth conditions. We have successfully broadened the hydroamination protocol to obtain various new E-divinylpiperazines with excellent regio- and stereoselectivities. The crystal structure of two of them was determined, which allowed receiving information, among others, on the conformation of the C–C double bond. Furthermore, the reaction allows facile access to enamines containing the isoindoline-1,3-dione fragment with high regioselectivity. Selected divinylpiperazines have also been investigated by photoluminescence excitation and emission examination. On the basis of the experimental results, the pathway of the catalytic hydroamination of alkyne with piperazine has been proposed.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


