Structure-Based Development of 3,4-Fused Tricyclic Benzofuran Derivatives as Polyketide Synthase 13 Inhibitors with Negligible hERG Inhibition
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
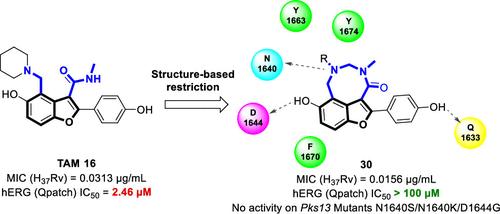
Polyketide synthase 13 (Pks13) is vital for synthesizing mycolic acid, which are essential for the survival of Mycobacterium tuberculosis (Mtb). Compounds that target Pks13 hold significant promise for developing new chemical entities for multidrug-resistant TB. The early lead benzofuran-3-carboxamide TAM16, demonstrated robust in vivo efficacy in murine models of tuberculosis infection; however, its further advancement was halted due to the cardiotoxicity associated with hERG inhibition. We implemented a conformational restriction strategy to explore the chemical space of 3,4-fused tricyclic benzofurans and indoles employing a structure-based design approach. Representative compounds were identified as Pks13-TE inhibitors, showing resistance against mutant strains from coumestan-resistant Mtb colonies. Notably, 29 and 30 exhibited potent antitubercular activity against Mtb H37Rv strain (MIC = 0.0156–0.0313 μg/mL), with negligible hERG inhibition (IC50 > 100 μM) suggesting that the 3,4-fused tricyclic benzofurans may present promising scaffold for developing Pks13-TE inhibitors without hERG liability.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


