CO2 Capture with Mg-, Al-, and Zr- Assisted CaO-Based Sorbents in the Calcium Looping Process Under Mild and Realistic Conditions
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
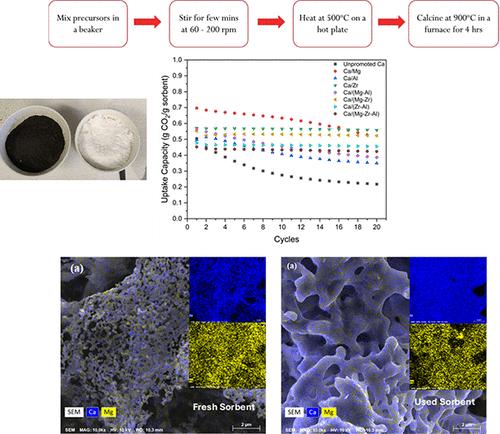
The calcium looping (CaL) process is a promising carbon capture technology for CO2 capture from point source emitters. A key challenge in the CaL process is the loss of sorbent capacity over successive capture-regeneration cycles due to sintering, which affects long-term stability. This study addresses this issue by a novel approach of incorporating MgO, Al2O3, and ZrO2 as promoters into calcium-based sorbents synthesized using the solution combustion synthesis (SCS) method. Sorbents were developed in mono-, bi-, and trimetallic configurations using soluble metal nitrates as precursors. Among the tested sorbents, Ca/(Zr–Al) demonstrated the highest CO2 uptake of 0.46 g of CO2/g of sorbent, while Ca/(Mg–Zr–Al) achieved 0.43 g of CO2/g of sorbent. Both configurations exhibited exceptional stability, maintaining over 90% of their initial capacity after 50 cycles at elevated temperatures. These results highlight the effectiveness of bi- and trimetallic sorbents in enhancing the performance and durability of the CaL process.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


