Bulk nanobubbles as active nucleation sites during antisovent crystallization
IF 4.3
2区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The control of crystallization is a critical yet challenging aspect of industrial processes, particularly in pharmaceuticals. In this work, we have hypothesized that remarkable stability of bulk nanobubbles may provide nucleation sites, control glycine crystal characteristics, and give high supersaturation regions for selective polymorph formation. The effect of different gaseous nanobubbles in various antisolvents were investigated. Nanobubbles were generated in the aqueous glycine solution by nanopore diffusion method. Our results indicate that nanobubbles influence crystal size distribution, with maximum reduction of 33 % in ethanol using oxygen nanobubbles. Nanobubbles also lead to more uniform crystals with an exclusive formation of the -polymorph. Gibbs free energy calculations show that nanobubbles decrease overall free energy, boosting nucleation rates. This action is attributed to glycine adsorption onto the negatively charged nanobubble surface. Overall, nanobubble technology proves to be a sustainable tool for regulating the crystallization process through efficient mixing of antisolvent and solute.
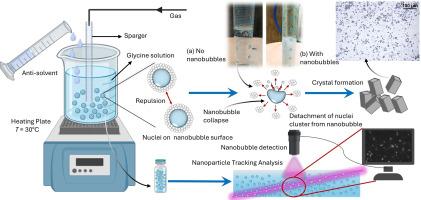
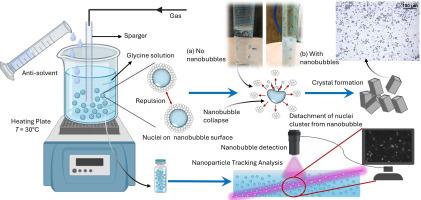
大块纳米气泡在抗溶剂结晶过程中的活性成核位置
结晶的控制是工业过程的一个关键但具有挑战性的方面,特别是在制药。在这项工作中,我们假设体积纳米气泡的显著稳定性可能提供成核位点,控制甘氨酸晶体特性,并为选择性多晶形成提供高过饱和区域。研究了不同气体纳米气泡在不同反溶剂中的作用。采用纳米孔扩散法在甘氨酸水溶液中制备了纳米气泡。研究结果表明,纳米气泡影响了乙醇的晶粒尺寸分布,在乙醇中使用氧纳米气泡可最大减少33%的晶粒尺寸。纳米气泡还导致更均匀的晶体与αα-多晶的独家形成。吉布斯自由能计算表明,纳米气泡降低了总自由能,提高了成核速率。这种作用归因于甘氨酸吸附在带负电荷的纳米泡表面。综上所述,纳米气泡技术是一种通过抗溶剂和溶质的有效混合来调节结晶过程的可持续工具。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Science
工程技术-工程:化工
CiteScore
7.50
自引率
8.50%
发文量
1025
审稿时长
50 days
期刊介绍:
Chemical engineering enables the transformation of natural resources and energy into useful products for society. It draws on and applies natural sciences, mathematics and economics, and has developed fundamental engineering science that underpins the discipline.
Chemical Engineering Science (CES) has been publishing papers on the fundamentals of chemical engineering since 1951. CES is the platform where the most significant advances in the discipline have ever since been published. Chemical Engineering Science has accompanied and sustained chemical engineering through its development into the vibrant and broad scientific discipline it is today.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


