Shear Stress-Responsive Peptide Cubic Vesicles Assembled from Membranes with Different Curvatures
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Stenotic blood vessels differ from normal blood vessels in that the blood flow shear stress is increased to a higher order of magnitude. Therefore, drug delivery systems (DDSs) capable of responding to changes in the shear stress are highly desirable. To prepare sheer stress-responsive carriers, a peptide cubic vesicle (PCV) is prepared by combining two types of sheet-forming amphiphilic polypeptides: planar sheet-forming GA-(PSar)10-b-(l-Leu-Aib)6-b-(PSar)10-GA (S10L12S10) and curved sheet-forming GA-(PSar)24-b-(l-Leu-Aib)7 (S26L14), which GA, PSar, Leu and Aib mean glycolic acid, polysarcosine, leucine and α-aminoisobutyric acid. The PCV is successfully constructed from a mixture of S10L12S10 and S26L14 in molar ratios of 2:1 and 1:1. In addition, curved S26L14 membrane forms edges and corners, while planar S10L12S10 membrane forms the faces of the PCV. Notably, the PCV deforms under pathological shear stress conditions (10 Pa) but retains its original structure under the normal physiological shearing force of 1 Pa. Moreover, the PCV releases 84% of its encapsulated cargo in response to simulated pathological flow. Targeting the changing biophysical environment for drug development has the potential to shift the paradigm for treating vascular occlusion-inducing diseases from biochemical to mechanical stimulation, thereby lowering the required dose and side effects of drugs while maximizing their therapeutic efficacy.
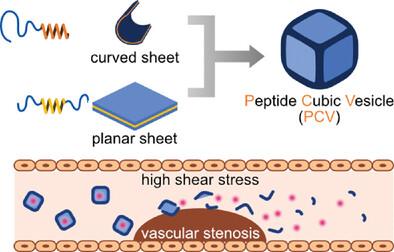
狭窄血管与正常血管的不同之处在于,血流剪应力会增加到更高的数量级。因此,能够对剪切应力的变化做出反应的药物输送系统(DDS)是非常理想的。为了制备剪切应力响应载体,我们将两种片状两亲多肽结合在一起,制备出了肽立方囊(PCV):平面片状多肽 GA-(PSar)10-b-(l-Leu-Aib)6-b-(PSar)10-GA (S10L12S10) 和弯曲片状多肽 GA-(PSar)24-b-(l-Leu-Aib)7 (S26L14),其中 GA、PSar、Leu 和 Aib 分别指乙醇酸、多肌氨酸、亮氨酸和α-氨基异丁酸。PCV 成功地由摩尔比为 2:1 和 1:1 的 S10L12S10 和 S26L14 混合物构建而成。此外,弧形的 S26L14 膜构成了 PCV 的边和角,而平面的 S10L12S10 膜则构成了 PCV 的面。值得注意的是,PCV 在病理剪切应力(10 Pa)条件下会变形,但在正常生理剪切力(1 Pa)条件下仍能保持原有结构。针对不断变化的生物物理环境进行药物开发,有可能将治疗血管闭塞诱发疾病的模式从生化刺激转变为机械刺激,从而降低药物的所需剂量和副作用,同时最大限度地提高疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


