Promotional role of boron on Ni/SiO2 catalysts for dibenzofuran hydrodeoxygenation
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
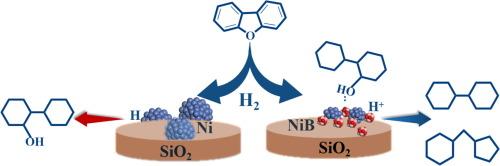
Controlling the oxygen-free product selectivity in catalytic hydrodeoxygenation is essential for producing high quality fuels from coal- and biomass-derived crude liquids. A series of boron-doped Ni/SiO2 catalysts were prepared for the efficient hydrodeoxygenation of dibenzofuran as a model compound. Boron doping significantly promoted the transformation of 2-cyclohexylcyclohexanol, which was accumulated as the main oxygen-containing intermediate compound on the pristine Ni/SiO2 catalyst, to the target products bicyclohexane and its isomer cyclopentylmethylcyclohexane. Characterization results show that boron doping increases Ni dispersion, modifies the electronic properties of Ni sites and in situ generates Brønsted acid sites by H2 spillover to boron oxides. The reduced Ni particle size improves the rates of dibenzofuran hydrogenation and 2-cyclohexylcyclohexanol dehydration. Electron-deficient Ni and boron oxides as the Lewis acid sites enhance the adsorption of 2-cyclohexylcyclohexanol. Furthermore, the in situ generated Brønsted acid sites favor the dehydration of 2-cyclohexylcyclohexanol, which gives the Ni-5B/SiO2 catalyst a better deoxygenation activity than the mechanical mixture of equal amounts of 5B/SiO2 and Ni/SiO2, as well as the one with a smaller Ni particle size of 3.8 nm.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


