Modulation of the Electronic Properties of Co3O4 through Bi Octahedral Doping for Enhanced Activity in the Oxygen Evolution Reaction
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
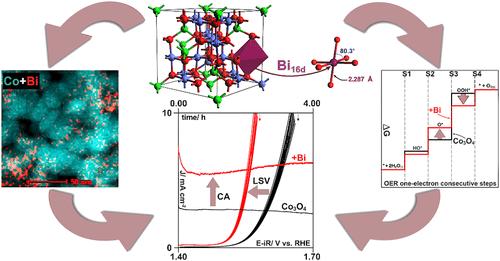
Developing a highly active and stable electrocatalyst for the oxygen evolution reaction (OER) is essential for efficient hydrogen production through anion exchange membrane water electrolysis powered by renewable electricity. Recently, there has been a renewed interest in designing electrocatalysts based on their work function optimization. The insights into the materials’ electronic properties gained from developing other heterogeneous catalysts, such as those used for N2O decomposition, can be thus leveraged to enhance the performance of the OER electrocatalysts. Knowing that Bi enhances the catalytic activity of Co3O4 in N2O decomposition, where the surface electronic properties play a crucial role, we hypothesized that it might also improve the electroactivity of the OER electroactivity. Therefore, we synthesized Bi-doped Co3O4 with different bismuth contents and studied the sample with a complementary set of physicochemical, electrochemical, and computational techniques. We found that promoting Co3O4 with atomically dispersed bismuth enhances its OER electrocatalytic properties by reducing the energy of the potential-determining step and improving electron charge transfer properties. Bismuth atoms enter octahedral sites in Co3O4, creating Bi active centers and enhancing the activity of vicinal Co sites in the OER. The Bi and modified Co centers are characterized by increased binding energy of the intermediate state of the metal–oxygen intermediate and increased density of states at the Fermi level. The former reduces the overpotential required for the OER, whereas the latter improves the reaction kinetics by decreasing the charge transfer resistance.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


