Fetal-like reversion in the regenerating intestine is regulated by mesenchymal asporin
IF 19.8
1区 医学
Q1 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Mesenchymal cells and the extracellular matrix (ECM) support epithelium during homeostasis and regeneration. However, the role of the mesenchyme in epithelial conversion into a fetal-like regenerative state after damage is not known. We modeled epithelial regeneration by culturing intestinal epithelium on decellularized small intestinal scaffolds (iECM) and identify asporin (Aspn), an ECM-bound proteoglycan, as a critical mediator of epithelial fetal-like reprogramming. After damage, transient increase in Aspn expression by the pericryptal fibroblasts induces epithelial transforming growth factor β (TGF-β)-signaling via CD44 and promotes timely epithelial reprogramming. Temporal control of Aspn is lost in old mice, and after damage, the persistently high level of Aspn stagnates epithelium in the regenerative state. Increase in Wnt signaling can resolve the stagnated regenerative program of the old epithelium, promoting restoration of tissue function. In summary, we establish a platform for modeling epithelial injury responses ex vivo and show that the mesenchymal Aspn-producing niche modulates tissue repair by regulating epithelial fetal-like reprogramming.
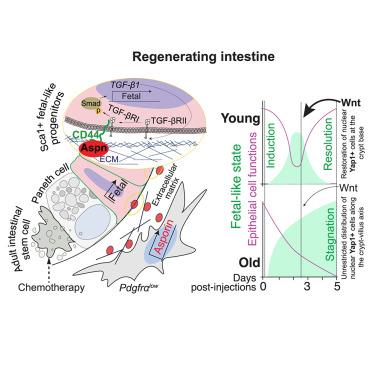
在再生肠中,胎儿样的逆转是由间充质间质酶调节的
间充质细胞和细胞外基质(ECM)在稳态和再生过程中支持上皮。然而,间充质在上皮细胞损伤后转化为胎儿样再生状态中的作用尚不清楚。我们通过在脱细胞化小肠支架(iECM)上培养肠上皮来模拟上皮再生,并鉴定了一种与脱细胞化小肠支架结合的蛋白聚糖——抗菌素(Aspn)是上皮类胎儿重编程的关键介质。损伤后,囊肿周围成纤维细胞短暂增加Aspn表达,通过CD44诱导上皮转化生长因子β (TGF-β)信号传导,促进上皮及时重编程。老年小鼠失去了对Aspn的时间控制,损伤后,Aspn的持续高水平使上皮停滞在再生状态。Wnt信号的增加可以解决老上皮细胞停滞的再生程序,促进组织功能的恢复。总之,我们建立了一个体外模拟上皮损伤反应的平台,并表明间充质aspn产生生态位通过调节上皮胎儿样重编程来调节组织修复。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell stem cell
生物-细胞生物学
CiteScore
37.10
自引率
2.50%
发文量
151
审稿时长
42 days
期刊介绍:
Cell Stem Cell is a comprehensive journal covering the entire spectrum of stem cell biology. It encompasses various topics, including embryonic stem cells, pluripotency, germline stem cells, tissue-specific stem cells, differentiation, epigenetics, genomics, cancer stem cells, stem cell niches, disease models, nuclear transfer technology, bioengineering, drug discovery, in vivo imaging, therapeutic applications, regenerative medicine, clinical insights, research policies, ethical considerations, and technical innovations. The journal welcomes studies from any model system providing insights into stem cell biology, with a focus on human stem cells. It publishes research reports of significant importance, along with review and analysis articles covering diverse aspects of stem cell research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


