Microglia dysfunction, neurovascular inflammation and focal neuropathologies are linked to IL-1- and IL-6-related systemic inflammation in COVID-19
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
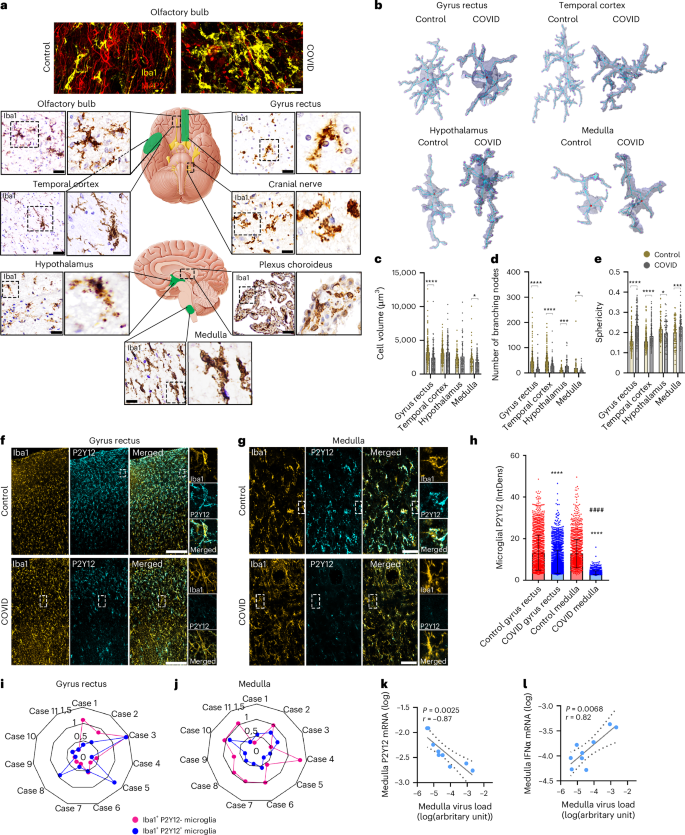
COVID-19 is associated with diverse neurological abnormalities, but the underlying mechanisms are unclear. We hypothesized that microglia, the resident immune cells of the brain, are centrally involved in this process. To study this, we developed an autopsy platform allowing the integration of molecular anatomy, protein and mRNA datasets in postmortem mirror blocks of brain and peripheral organ samples from cases of COVID-19. We observed focal loss of microglial P2Y12R, CX3CR1–CX3CL1 axis deficits and metabolic failure at sites of virus-associated vascular inflammation in severely affected medullary autonomic nuclei and other brain areas. Microglial dysfunction is linked to mitochondrial injury at sites of excessive synapse and myelin phagocytosis and loss of glutamatergic terminals, in line with proteomic changes of synapse assembly, metabolism and neuronal injury. Furthermore, regionally heterogeneous microglial changes are associated with viral load and central and systemic inflammation related to interleukin (IL)-1 or IL-6 via virus-sensing pattern recognition receptors and inflammasomes. Thus, SARS-CoV-2-induced inflammation might lead to a primarily gliovascular failure in the brain, which could be a common contributor to diverse COVID-19-related neuropathologies. The authors show that brain inflammation in COVID-19 correlates with viral load, systemic inflammation and virus-sensing pattern recognition receptors. Microglial dysfunction occurs at sites of vascular inflammation with myelin injury and synapse loss.

小胶质细胞功能障碍、神经血管炎症和局灶性神经病变与COVID-19中IL-1和il -6相关的全身性炎症有关
COVID-19与多种神经异常有关,但潜在机制尚不清楚。我们假设小胶质细胞,大脑的常驻免疫细胞,在这个过程中起着核心作用。为了研究这一点,我们开发了一个尸检平台,可以整合来自COVID-19病例的脑和外周器官样本的死后镜像块中的分子解剖学、蛋白质和mRNA数据集。我们观察到在严重受影响的髓系自主神经核和其他脑区病毒相关血管炎症部位,小胶质细胞P2Y12R、CX3CR1-CX3CL1轴缺陷和代谢衰竭的局灶性丧失。小胶质细胞功能障碍与突触过度、髓磷脂吞噬和谷氨酸末端缺失部位的线粒体损伤有关,与突触组装、代谢和神经元损伤的蛋白质组学变化一致。此外,区域异质性的小胶质细胞变化与病毒载量以及通过病毒感知模式识别受体和炎症小体与白细胞介素(IL)-1或IL-6相关的中枢和全身性炎症有关。因此,sars - cov -2诱导的炎症可能导致大脑主要的胶质血管衰竭,这可能是导致各种与covid -19相关的神经病变的共同因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


