Recompensation of decompensated cirrhosis in hepatitis C patients after SVR: Prognostic implications
IF 26.8
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Backgound&Aims
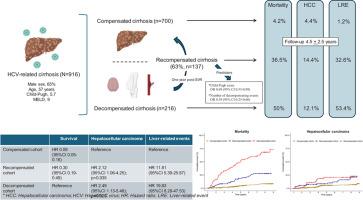
The Baveno VII consensus introduced the term “recompensated cirrhosis,” though few studies have examined its clinical relevance. We analyzed the rate and predictors of recompensation in hepatitis C (HCV) patients after sustained virological response (SVR) and evaluated its impact on mortality and hepatocellular carcinoma (HCC) compared to compensated and decompensated cirrhosis.Methods
Multicenter observational study enrolling 916 HCV-related cirrhotic patients, with a minimum follow-up period of 12 months after SVR. Subjects were categorized into three mutually exclusive groups: compensated, decompensated, and recompensated group. Patients were followed until the occurrence of liver transplantation, death, or the last follow-up date, whichever came first.Results
During the study (4.5±2.5 years), 12% (110/916) experienced a decompensating event, 7.7% (71/916) were diagnosed with HCC, and 14.9% (137/916) died. Among 23% (216/916) patients who were decompensated at baseline, 63.4% (137/216) achieved recompensation at 12 months. Child-Pugh score [OR 0.69 (95% CI 0.53-0.89); p=0.005] and the number of past decompensating events were associated with recompensation. The compensated cohort exhibited a lower mortality rate (4.2% (28/663)) than recompensated (36.5% (50/137)), and decompensated subjects (50% (30/60)) (p=0.0001). Along with age [CSHR 1.03 (95% CI 1.01-1.05); p=0.0009], albumin [CSHR 0.67 (95% CI 0.45-0.98); p=0.038], INR, [CSHR 1.88 (95% CI 1.14-3.10); p=0.014], bilirubin levels [CSHR 1.28 (95% CI 1.08-1.50);p=0.003], recompensated [CSHR 0.30 (95% CI 0.19-0.49); p=0.0001] and compensated states [CSHR 0.09 (95% CI 0.05-0.16); p=0.0001] were associated with mortality. By contrast, HCC occurrence was significantly lower in compensated (4.4% (29/662)) than recompensated (14.4% (19/132)), and decompensated patients (12.1% (7/58)) (p=0.0001).Conclusions
Two-thirds of patients with decompensated cirrhosis achieved recompensation twelve months post-SVR, leading to improved survival compared to those without recompensation, though still lower than in compensated patients. However, HCC risk remained unchanged in the recompensated cohort.
SVR后丙型肝炎失代偿肝硬化的再代偿:预后意义
背景和目的 巴韦诺第七次共识会议提出了 "再代偿肝硬化 "这一术语,但很少有研究探讨其临床意义。我们分析了持续病毒学应答(SVR)后丙型肝炎(HCV)患者再代偿的比率和预测因素,并评估了与代偿性和失代偿性肝硬化相比,再代偿对死亡率和肝细胞癌(HCC)的影响。研究对象被分为三个互斥组:代偿组、失代偿组和再代偿组。研究期间(4.5±2.5 年),12%(110/916)的患者出现失代偿,7.7%(71/916)的患者被诊断为 HCC,14.9%(137/916)的患者死亡。在基线失代偿的 23% (216/916)患者中,63.4% (137/216)的患者在 12 个月后恢复了代偿。Child-Pugh评分[OR 0.69 (95% CI 0.53-0.89); p=0.005]和既往失代偿事件的次数与代偿相关。代偿组的死亡率(4.2% (28/663))低于再代偿组(36.5% (50/137))和失代偿组(50% (30/60))(P=0.0001)。与年龄[CSHR 1.03 (95% CI 1.01-1.05); p=0.0009]、白蛋白[CSHR 0.67 (95% CI 0.45-0.98); p=0.038]、INR、[CSHR 1.88 (95% CI 1.14-3.10); p=0.014]、胆红素水平[CSHR 1.28(95% CI 1.08-1.50);p=0.003]、再代偿[CSHR 0.30(95% CI 0.19-0.49);p=0.0001]和代偿状态[CSHR 0.09(95% CI 0.05-0.16);p=0.0001]与死亡率相关。相比之下,代偿期患者(4.4% (29/662))的 HCC 发生率明显低于代偿期患者(14.4% (19/132))和失代偿期患者(12.1% (7/58))(P=0.0001)。然而,失代偿组群的 HCC 风险保持不变。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hepatology
医学-胃肠肝病学
CiteScore
46.10
自引率
4.30%
发文量
2325
审稿时长
30 days
期刊介绍:
The Journal of Hepatology is the official publication of the European Association for the Study of the Liver (EASL). It is dedicated to presenting clinical and basic research in the field of hepatology through original papers, reviews, case reports, and letters to the Editor. The Journal is published in English and may consider supplements that pass an editorial review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


