Activating Carbon and Oxygen Bonds for Low-Temperature Thermal Decomposition of Spent Lithium-Ion Battery Cathode Materials
IF 10.8
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
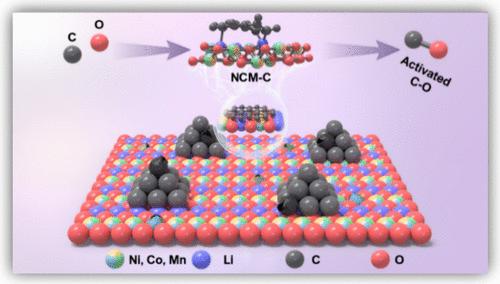
The temperature for complete disintegration of spent lithium-ion battery (LIB) cathode materials is typically in a range of 750–1400 °C, resulting in intensive energy consumption and high carbon emissions. Here, we promote the bond activation of oxygen in LiNi0.5Co0.2Mn0.3O2 and carbon in graphite electrodes, achieving rapid gasification and thermal decomposition of active crystals at lower temperatures in the absence of other activating agents. The activation of C and O bond leads to the storage of internal energy and the transition of the crystalline phase (single crystal to polycrystal) of the active crystals. Density functional theory modeling confirms that the CO adsorption energy is significantly higher with Ca–Oa (−3.35 eV, C and O activation) than with no activation (−1.66 eV). The differential charge results show that the bond activation model has the highest charge accumulation and consumption, improving the electron transfer. The Bader charge transfer between Ca–Oa and CO is also the largest, with a value of 0.433 |e|. Therefore, synchronous activation of C and O bonds can reduce the decomposition temperature of active crystals by 200 °C and allows a low-temperature pyrolysis recycling of retired LIB cathode materials. Our research provides a potential strategy for low-carbon recycling of retired LIBs worldwide.
求助全文
约1分钟内获得全文
求助全文
来源期刊

环境科学与技术
环境科学-工程:环境
CiteScore
17.50
自引率
9.60%
发文量
12359
审稿时长
2.8 months
期刊介绍:
Environmental Science & Technology (ES&T) is a co-sponsored academic and technical magazine by the Hubei Provincial Environmental Protection Bureau and the Hubei Provincial Academy of Environmental Sciences.
Environmental Science & Technology (ES&T) holds the status of Chinese core journals, scientific papers source journals of China, Chinese Science Citation Database source journals, and Chinese Academic Journal Comprehensive Evaluation Database source journals. This publication focuses on the academic field of environmental protection, featuring articles related to environmental protection and technical advancements.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


