Targeting the mosquito prefoldin–chaperonin complex blocks Plasmodium transmission
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
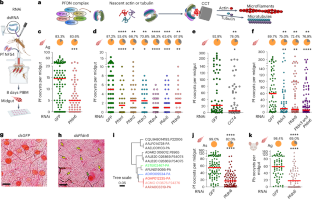
The Plasmodium infection cycle in mosquitoes relies on numerous host factors in the vector midgut, which can be targeted with therapeutics. The mosquito prefoldin complex is needed to fold proteins and macromolecular complexes properly. Here we show that the conserved Anopheles mosquito prefoldin (PFDN)–chaperonin system is a potent transmission-blocking target for multiple Plasmodium species. Silencing any prefoldin subunit or its CCT/TRiC partner via RNA interference reduces Plasmodium falciparum oocyst loads in the mosquito midgut, as does co-feeding mosquitoes with PFDN6-specific antibody and gametocytes. Inhibition of the PFDN–CCT/TRiC chaperonin complex results in the loss of epithelial and extracellular matrix integrity, which triggers microorganism-mediated anti-Plasmodium immune priming and compromises the parasite’s laminin-based immune evasion. Mouse malaria transmission-blocking vaccine and antibody co-feeding assays support its potential as a multispecies transmission-blocking target for P. falciparum and Plasmodium vivax. Further study is needed to determine the potential of this system as a transmission-blocking vaccine target. Disrupting the prefoldin–chaperonin complex in various species of Anopheles mosquitoes results in a compromised intestinal barrier leading to an immune response that inhibits the malaria parasite.

靶向蚊子前折叠蛋白-伴侣蛋白复合物阻断疟原虫传播
蚊子的疟原虫感染周期依赖于媒介中肠中的许多宿主因子,这些因子可以作为治疗的目标。蚊子的前折叠蛋白复合体是折叠蛋白质和大分子复合体所必需的。本研究表明,保守的按蚊预折叠蛋白(PFDN) -伴侣蛋白系统是多种疟原虫的有效传播阻断靶点。通过RNA干扰沉默任何前折叠蛋白亚基或其CCT/TRiC伴侣可减少蚊子中肠中的恶性疟原虫卵囊负荷,与pfdn6特异性抗体和配子细胞共同喂养蚊子也是如此。PFDN-CCT /TRiC伴侣蛋白复合物的抑制导致上皮和细胞外基质完整性的丧失,从而触发微生物介导的抗疟原虫免疫启动,并损害寄生虫基于层粘连蛋白的免疫逃避。小鼠疟疾传播阻断疫苗和抗体共饲喂试验支持其作为恶性疟原虫和间日疟原虫多物种传播阻断靶点的潜力。需要进一步研究以确定该系统作为传播阻断疫苗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


