Multi-omics clustering analysis carries out the molecular-specific subtypes of thyroid carcinoma: implicating for the precise treatment strategies
IF 4.5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
Thyroid cancer (TC) is the most prevalent endocrine malignancy worldwide. This study aimed to explore the molecular subtypes and improve the selection of targeted therapies. We used multi-omics data from 539 patients with DNA methylation, gene mutations, mRNA, lncRNA, and miRNA expressions. This study employed consensus clustering algorithms to identify molecular subtypes and used various bioinformatics tools to analyze genetic alterations, signaling pathways, immune infiltration, and responses to chemotherapy and immunotherapy. Two prognostically relevant TC subtypes, CS1 and CS2, were identified. CS2 was associated with a poorer prognosis of shorter progression-free survival times (P < 0.001). CS1 exhibited higher copy number alterations but a lower tumor mutation burden than CS2. CS2 exhibited activation in cell proliferation and immune-related pathways. Drug sensitivity analysis indicated CS2’s higher sensitivity to cisplatin, doxorubicin, paclitaxel, and sunitinib, whereas CS1 was more sensitive to bicalutamide and FH535. The different activated pathways and sensitivity to drugs for the subtypes were further validated in an external cohort. Twenty-four paired tumors and adjacent normal tissues by immunohistochemical staining further demonstrated the prognostic value of CXCL17. In conclusion, we identified two distinct molecular subtypes of TC with significant implications for prognosis, genetic alterations, pathway activation, and treatment response.
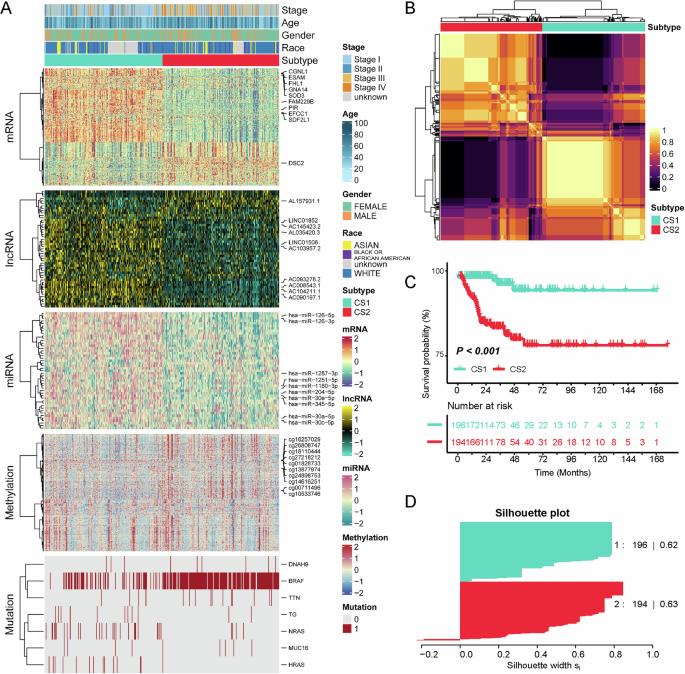
多组学聚类分析对甲状腺癌的分子特异性亚型进行分析,为精确的治疗策略提供依据。
甲状腺癌是世界上最常见的内分泌恶性肿瘤。本研究旨在探索分子亚型,提高靶向治疗的选择。我们使用了来自539例DNA甲基化、基因突变、mRNA、lncRNA和miRNA表达的患者的多组学数据。本研究采用共识聚类算法来识别分子亚型,并使用各种生物信息学工具来分析遗传改变、信号通路、免疫浸润以及对化疗和免疫治疗的反应。两种与预后相关的TC亚型CS1和CS2被确定。CS2与预后较差、无进展生存时间较短相关(P
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


