VIPER-TACs leverage viral E3 ligases for disease-specific targeted protein degradation
IF 7.2
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
In targeted protein degradation (TPD) a protein of interest is degraded by chemically induced proximity to an E3 ubiquitin ligase. One limitation of using TPD therapeutically is that most E3 ligases have broad tissue expression, which can contribute to toxicity via target degradation in healthy cells. Many pathogenic and oncogenic viruses encode E3 ligases (vE3s), which de facto have strictly limited expression to diseased cells. Here, we provide proof-of-concept for viral E3 pan-essential removing targeting chimeras (VIPER-TACs) that are bi-functional molecules that utilize viral E3 ubiquitin ligases to selectively degrade pan-essential proteins and eliminate diseased cells. We find that the human papillomavirus (HPV) ligase E6 can degrade the SARS1 pan-essential target protein in a model of HPV-positive cervical cancer to selectively kill E6 expressing cancer cells. Thus, VIPER-TACs have the capacity to dramatically increase the therapeutic window, alleviate toxicity concerns, and ultimately expand the potential target space for TPD.

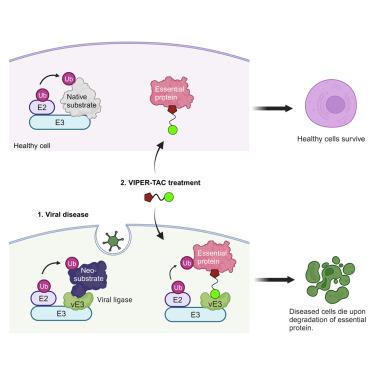
viper - tac利用病毒E3连接酶进行疾病特异性靶向蛋白降解
在靶向蛋白降解(TPD)中,感兴趣的蛋白通过化学诱导接近E3泛素连接酶而降解。使用TPD治疗的一个限制是,大多数E3连接酶具有广泛的组织表达,这可能通过健康细胞中的靶标降解导致毒性。许多致病性和致癌性病毒编码E3连接酶(vE3s),实际上严格限制其在病变细胞中的表达。在这里,我们提供了病毒E3泛必要去除靶向嵌合体(viper - tac)的概念证明,该嵌合体是双功能分子,利用病毒E3泛必要蛋白连接酶选择性地降解泛必要蛋白并消除病变细胞。我们发现人乳头瘤病毒(HPV)连接酶E6可以降解人乳头瘤病毒(HPV)阳性宫颈癌模型中SARS1泛必需靶蛋白,选择性杀死表达E6的癌细胞。因此,viper - tac有能力显著增加治疗窗口,减轻毒性担忧,并最终扩大TPD的潜在靶点空间。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Chemical Biology
Biochemistry, Genetics and Molecular Biology-Molecular Medicine
CiteScore
14.70
自引率
2.30%
发文量
143
期刊介绍:
Cell Chemical Biology, a Cell Press journal established in 1994 as Chemistry & Biology, focuses on publishing crucial advances in chemical biology research with broad appeal to our diverse community, spanning basic scientists to clinicians. Pioneering investigations at the chemistry-biology interface, the journal fosters collaboration between these disciplines. We encourage submissions providing significant conceptual advancements of broad interest across chemical, biological, clinical, and related fields. Particularly sought are articles utilizing chemical tools to perturb, visualize, and measure biological systems, offering unique insights into molecular mechanisms, disease biology, and therapeutics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


