Assembling Ruthenium Complexes to Form Ruthenosome Unleashing Ferritinophagy-Mediated Tumor Suppression
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We introduce ruthenosomes, a fusion of liposomal and reactive oxygen species (ROS)–generating properties meticulously engineered as potent ferroptosis inducers (FINs), marking a significant advancement in metallodrug design for cancer therapy. Formed through the self-assembly of oleate-conjugated ruthenium complexes, these ruthenosomes exhibit exceptional cellular uptake, selectively accumulating in mitochondria and causing substantial disruption. This targeted mitochondrial damage significantly elevates ROS levels, triggering autophagy and selectively activating ferritinophagy. Together, these processes sensitize cancer cells to ferroptosis. In vivo, ruthenosomes effectively suppress colorectal tumor growth, underscoring their therapeutic potential. Our study pioneers a design strategy that transforms ruthenium complexes into liposome-like structures capable of inducing ferroptosis independent of light activation. By leveraging ruthenosomes as multifunctional nanocarriers, this research offers a versatile and powerful platform for ROS-mediated, ferroptosis-driven cancer cell eradication.
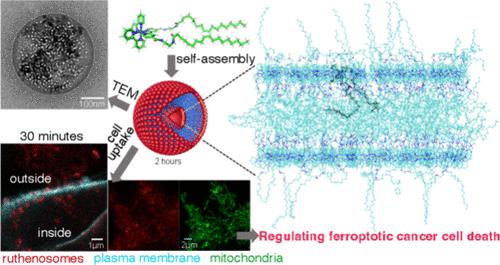
组装钌复合物形成钌小体,释放噬铁蛋白介导的肿瘤抑制能力
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


