Quercetin–Copper Complexation-Based Porous Polymer for Chromium, Mercury, and Cadmium Metal Ion Adsorption: Experimental and Computational Study
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
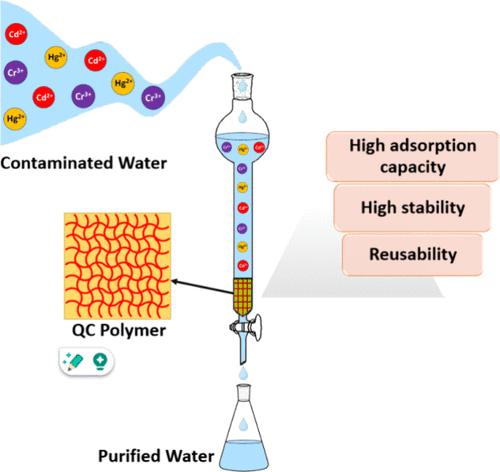
Heavy metal ions in water bodies pose a serious threat to human health and the environment. To overcome this issue, we developed a polymeric quercetin–copper complex (QC) as a novel adsorbent for heavy metal removal. This material was characterized using different techniques. Its structure was also investigated using density functional theory (DFT) calculations, which revealed the formation of a highly stable 2:1 complex between quercetin and Cu2+ ion. The QC polymer exhibited >95% adsorption efficiency for removing heavy metals under acidic and neutral pH conditions. The adsorption capacities were 2.50, 0.77, and 0.54 mmol/g for Cr3+, Hg2+, and Cd2+ ions, respectively, under neutral pH conditions. DFT calculations also indicated high adsorption energies of −34.32, −15.10, and −12.37 eV for Cr3+, Hg2+, and Cd2+, respectively. The QC polymer was easily recovered and reused for removal studies of these metal ions without showing a significant loss in its adsorption capacity.
基于槲皮素-铜络合的多孔聚合物对铬、汞和镉金属离子的吸附:实验与计算研究
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


