Nucleation Thermodynamics and Nucleation Kinetics of Ammonium Sulfate under the Synergistic Action of Ammonium Chloride and Ammonium Fluoride
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
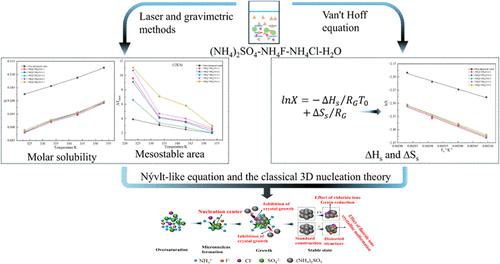
In order to investigate the nucleation thermodynamics and nucleation kinetics of (NH4)2SO4 during cooling crystallization under the synergistic effect of different ratios of NH4F and NH4Cl, the solubility and width of the metastable zone width of (NH4)2SO4 in the mixed solution were determined by gravimetric and laser methods by incorporation of (NH4)2SO4 in a mixture of different ratios of NH4F and NH4Cl in this study. It is demonstrated that the dissolution of (NH4)2SO4 is a heat-absorbing process, that the inhibition of (NH4)2SO4 nucleation by NH4F is much greater, and that the intermediate stabilization zone of (NH4)2SO4 is enlarged with an increase in the cooling rate. Based on the self-consistent Nývlt-like equation and the classical three-dimensional nucleation theory, the nucleation kinetics of (NH4)2SO4 under the synergistic interaction of NH4F and NH4Cl was investigated, and the critical nucleation parameters of (NH4)2SO4 under the synergistic interaction of NH4F and NH4Cl were obtained.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


