Immobilization and release behaviors of uranium mediated by the redox processes between manganese oxides and dissolved organic matter: Effects of pH and goethite
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
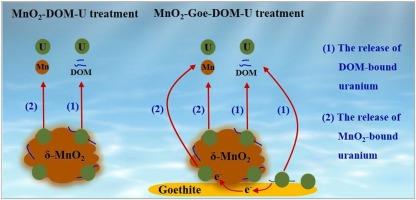
Both manganese dioxide (MnO₂) and dissolved organic matter (DOM) exert a significant influence on the chemical species of uranium in the contaminated soils, yet the impacts of the interactions between MnO2 and DOM, particularly in the presence of iron oxyhydroxides, on the environmental behaviors of uranium have not been elucidated. In this study, the dynamic behaviors of uranium were investigated during the reactions of DOM with δ-MnO2 in the presence of goethite at different pH values, by employing a combination of kinetic experiments, spectrophotometric titration, X-ray photoelectron spectroscopy, and electrochemical analysis. Our results indicated that the presence of DOM decreased uranium adsorption on MnO2 and promoted the release of uranium bound to DOM and MnO2 through the oxidation of DOM and the reduction of MnO2, respectively. Goethite increased uranium adsorption on its surface and hindered the direct oxidation of DOM by MnO2, but the indirect oxidation of goethite-adsorbed DOM by MnO2 provided an additional route for uranium release. We found that uranium concentration in solution was positively correlated with Mn(II) concentration at pH 4.5, whereas it was positively correlated with the concentration of dissolved organic carbon and negatively correlated with the aromaticity and molecular weight of DOM at pH 6.5. Above results highlighted the significance of the redox process between MnO2 and DOM in regulating the dynamic behaviors of uranium, which contributed to a better understanding of the sequestration and stability of uranium in the contaminated soils around the uranium tailings ponds.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


