Hole Trapping in Lead Halide Perovskite Nanocrystal–Viologen Hybrids and Its Impact on Back Electron Transfer
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
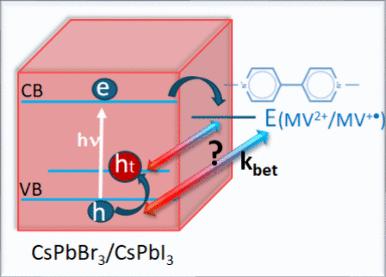
Control of forward and back electron transfer processes in semiconductor nanocrystals is important to maximize charge separation for photocatalytic reduction/oxidation processes. By employing methyl viologen as the electron acceptor, we have succeeded in mapping the electron transfer from excited CsPbI3 nanocrystals to viologen as well as the hole trapping process. The electron transfer to viologen is an ultrafast process (ket = 2 × 1010 s–1) and results in the formation of extended charge separation as electrons are trapped at surface-bound viologen sites and holes at iodide sites. The I2─• formation, which is confirmed through the transient absorption at 750 nm, provides a convenient way to probe trapped holes and its participation in the back electron transfer process. By employing a series of mixed halide compositions, we were able to tune the bandgap and valence band energy of the perovskite donor. The back electron transfer rate constant (kbet = 1.3–2.6 × 107 s–1) is nearly three orders of magnitude smaller than that of forward electron transfer, thus extending the lifetime of the charge-separated state. The weak dependence of the back electron transfer rate constant on the valence band energy suggests that trapping of holes at halide (I or Br) sites is involved in the back electron transfer process. The ability to extend the lifetime of the charge-separated pair can offer new strategies to improve the redox properties of semiconductor-based photocatalytic systems.
卤化铅过氧化物纳米晶-紫胶杂化物中的空穴捕获及其对反向电子转移的影响
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


