Oxygen Atom Transfer Reactions of Colloidal Metal Oxide Nanoparticles
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
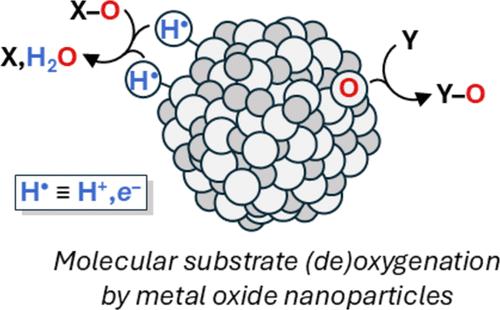
Redox transformations at metal oxide (MOx)/solution interfaces are broadly important, and oxygen atom transfer (OAT) is one of the simplest and most fundamental examples of such reactivity. OAT is a two-electron transfer process, well-known in gas/solid reactions and catalysis. However, OAT is rarely directly observed at oxide/water interfaces, whose redox reactions are typically proposed to occur in one-electron steps. Reported here are stoichiometric OAT reactions of organic molecules with aqueous colloidal titanium dioxide and iridium oxide nanoparticles (TiO2 and IrOx NPs). Me2SO (DMSO) oxidizes reduced TiO2 NPs with the formation of Me2S, and IrOx NPs transfer O atoms to a water-soluble phosphine and a thioether. The reaction stoichiometries were established and the chemical mechanisms were probed using typical solution spectroscopic techniques, exploiting the high surface areas and transparency of the colloids. These OAT reactions, including a catalytic example, utilize the ability of the individual NPs to accumulate many electrons and/or holes. Observing OAT reactions of two different materials, in opposite directions, is a step toward harnessing oxide nanoparticles for valuable multi-electron and multi-hole transformations.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


