Heterointerface with Continuous Channels Enables Fast Na+ Transport in Layered Na2Ti3O7
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
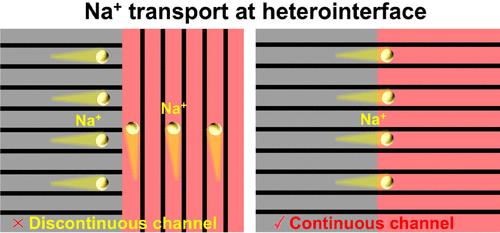
High-power sodium-ion batteries are essential for grid energy storage; however, they are generally limited by Na+ transport. Herein, we tailor a highly matched heterostructure (MgTi3O7@Na2Ti3O7) via a facile in situ synthesis method. The similar crystal structures of Na2Ti3O7 and MgTi3O7 creat continuous Na+ diffusion channels at the heterointerface, and the interactions at the interface creat a built-in interface electric field with a direction from MgTi3O7 to Na2Ti3O7. As a result, the particular heterointerface enable rapid Na+ diffusion in the MgTi3O7@Na2Ti3O7 electrode. The heterostructure engineering regulate the electrochemical reaction mechanism, leading to the solid solution reaction in the MgTi3O7@Na2Ti3O7 electrode, facilitating rapid Na+ transport. Therefore, the MgTi3O7@Na2Ti3O7 electrode exhibits an excellent rate capability (123 mAh/g at 20 C) and cycling performance. This work highlights the importance of a heterointerface with continuous channels in overcoming Na+ transport limitations in electrodes and could serves as a guide for designing a heterointerface for high-power sodium-ion batteries.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


