Chanoclavine synthase operates by an NADPH-independent superoxide mechanism
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
More than ten ergot alkaloids comprising both natural and semi-synthetic products are used to treat various diseases1,2. The central C ring forms the core pharmacophore for ergot alkaloids, giving them structural similarity to neurotransmitters, thus enabling their modulation of neurotransmitter receptors3. The haem catalase chanoclavine synthase (EasC) catalyses the construction of this ring through complex radical oxidative cyclization4. Unlike canonical catalases, which catalyse H2O2 disproportionation5,6, EasC and its homologues represent a broader class of catalases that catalyse O2-dependent radical reactions4,7. We have elucidated the structure of EasC by cryo-electron microscopy, revealing a nicotinamide adenine dinucleotide phosphate (reduced) (NADPH)-binding pocket and a haem pocket common to all haem catalases, with a unique homodimeric architecture that is, to our knowledge, previously unobserved. The substrate prechanoclavine unprecedentedly binds in the NADPH-binding pocket, instead of the previously suspected haem-binding pocket, and two pockets were connected by a slender tunnel. Contrary to the established mechanisms, EasC uses superoxide rather than the more generally used transient haem iron–oxygen complexes (such as compounds I, II and III)8,9, to mediate substrate transformation through superoxide-mediated cooperative catalysis of the two distant pockets. We propose that this reactive oxygen species mechanism could be widespread in metalloenzyme-catalysed reactions. The unique structure and mechanism of chanoclavine synthase (EasC), a haem catalase that uses superoxide for substrate transformation in ergot alkaloid biosynthesis, are revealed in this study, challenging established catalase mechanisms.

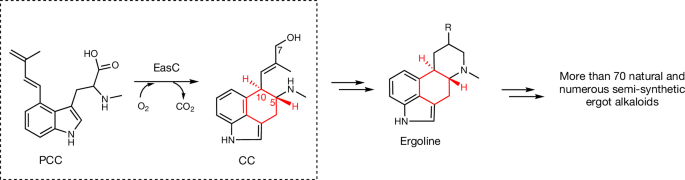
蛇碱合酶通过nadph不依赖的超氧化物机制起作用
包括天然和半合成产品的十多种麦角生物碱被用于治疗各种疾病1,2。中央的C环构成了麦角生物碱的核心药效团,使其在结构上与神经递质相似,从而能够调节神经递质受体3。血红素过氧化氢酶chanoclavine synthase (EasC)通过复杂的自由基氧化环化催化该环的构建4。与催化H2O2歧化的典型过氧化氢酶5,6不同,EasC及其同源物代表了催化o2依赖自由基反应的更广泛的过氧化氢酶类别4,7。我们已经通过低温电子显微镜阐明了EasC的结构,揭示了所有血红素过氧化氢酶共有的烟酰胺腺嘌呤二核苷酸磷酸(NADPH)结合袋和血红素袋,具有独特的同二聚体结构,据我们所知,这是以前未观察到的。底物前棘突史无前例地结合在nadph结合口袋中,而不是之前怀疑的血液结合口袋,两个口袋通过一条细长的隧道连接起来。与已建立的机制相反,EasC使用超氧化物而不是更普遍使用的瞬态血红素铁氧配合物(如化合物I, II和III)8,9,通过超氧化物介导的两个远端口袋的协同催化来介导底物转化。我们认为这种活性氧机制可能在金属酶催化反应中广泛存在。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


