Probing Temperature Effect on Enhanced Electrochemical CO2 Reduction of Hydrophobic Au25(SR)18 Nanoclusters
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The widely studied electrocatalytic CO2 reduction reaction (eCO2RR) has typically been operated at room temperature. However, practical electrolyzers might operate at elevated temperatures, but a major concern is low CO2 solubility. One promising strategy is to construct a hydrophobic interface to enhance CO2 diffusion. Regarding this, atomically precise gold nanoclusters (NCs) can be accurately decorated with hydrophobic ligands to create a local hydrophobic microenvironment to ensure rapid CO2 transfer, yet the temperature effect on the reaction kinetics remains unknown. Here, we report, for the first time, the temperature-dependent eCO2RR performance of hydrophobic Au25(SR)18 NCs by a close interplay between theory and experiment. Simulations revealed that the hydrophobic surface is very conducive to CO2 activation, and the proton transfer process for *COOH and *CO formation can be significantly affected by temperature via modulating interface hydrogen bonding. Particularly, an elevated temperature at 330 K dramatically increases the catalytic activity while simultaneously suppressing the competitive hydrogen evolution reaction. We experimentally demonstrate that Au25 exhibits high eCO2RR performance at 330 K, achieving a high CO Faradaic efficiency of ∼93% and a CO partial current density about 2 times higher than that at room temperature. This work opens exciting opportunities in developing efficient electrocatalysts via synergistic implementation of surface hydrophobicity and temperature-mediated interface engineering.
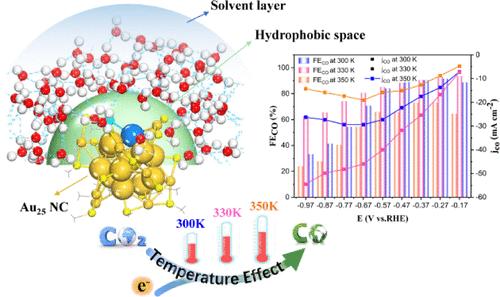
探究温度对疏水性 Au25(SR)18 纳米团簇增强电化学二氧化碳还原的影响
广泛研究的电催化CO2还原反应(eCO2RR)通常在室温下进行。然而,实用的电解槽可能在高温下运行,但主要问题是二氧化碳溶解度低。一个有希望的策略是构建疏水界面来增强CO2扩散。因此,原子精度的金纳米簇(NCs)可以用疏水配体精确修饰,以创建局部疏水微环境,以确保快速的CO2转移,但温度对反应动力学的影响尚不清楚。在这里,我们首次通过理论和实验的密切相互作用,报道了疏水性Au25(SR)18纳米材料的eCO2RR性能随温度的变化。模拟结果表明,疏水表面非常有利于CO2的活化,通过调节界面氢键,温度可以显著影响*COOH和*CO形成的质子转移过程。特别是,在330 K温度升高时,催化活性显著提高,同时抑制了竞争性析氢反应。实验证明,在330 K下,Au25具有很高的eCO2RR性能,CO法拉第效率高达93%,CO分电流密度比室温下高约2倍。这项工作为通过表面疏水性和温度介导界面工程的协同实现开发高效电催化剂提供了令人兴奋的机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


