Rapid and Scalable Synthesis of Oxazoles Directly from Carboxylic Acids
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
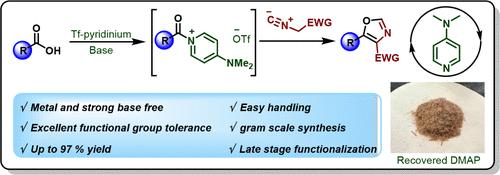
A highly efficient and expedient method for the synthesis of 4,5-disubstituted oxazoles has been developed directly from carboxylic acids, employing a stoichiometric amount of the easy-to-access and stable triflylpyridinium reagent. The overall transformation proceeds through the formation of an in situ generated acylpyridinium salt followed by trapping with isocyanoacetates and tosylmethyl isocyanide. This transformation has a broad substrate scope with good functional group tolerance (including hindered and less reactive substrates or those containing sensitive functional groups). The versatility of this newly developed reaction is illustrated through its application in the gram-scale production of the FDA-approved prodrug 5-aminolevulinic acid (5-ALA) and the late-stage functionalization of bioactive molecules including estrone, lipoic acid, valproic acid, and probenecid. Additionally, this process features the advantageous recovery and reuse of the base DMAP, underscoring its practical benefits.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


